Abstract
We previously reported that the 18-mer amphiphilic α-helical peptide, Hel 13-5, consisting of 13 hydrophobic residues and five hydrophilic amino acid residues, can induce neutral liposomes (egg yolk phosphatidylcholine) to adopt long nanotubular structures and that the interaction of specific peptides with specific phospholipid mixtures induces the formation of membrane structures resembling cellular organelles such as the Golgi apparatus. In the present study we focused our attention on the effects of peptide sequence and chain length on the nanotubule formation occurring in mixture systems of Hel 13-5 and various neutral and acidic lipid species by means of turbidity measurements, dynamic light scattering measurements, and electron microscopy. We designed and synthesized two sets of Hel 13-5 related peptides: 1) Five peptides to examine the role of hydrophobic or hydrophilic residues in amphiphilic α-helical structures, and 2) Six peptides to examine the role of peptide length, having even number residues from 12 to 24. Conformational, solution, and morphological studies showed that the amphiphilic α-helical structure and the peptide chain length (especially 18 amino acid residues) are critical determinants of very long tubular structures. A mixture of α-helix and β-structures determines the tubular shapes and assemblies. However, we found that the charged Lys residues comprising the hydrophilic regions of amphiphilic structures can be replaced by Arg or Glu residues without a loss of tubular structures. This suggests that the mechanism of microtubule formation does not involve the charge interaction. The immersion of the hydrophobic part of the amphiphilic peptides into liposomes initially forms elliptic-like structures due to the fusion of small liposomes, which is followed by a transformation into tubular structures of various sizes and shapes.
INTRODUCTION
The control of material architecture on the nanometer scale has recently emerged as an important field in both biology and technology. Indeed, nanotubular structures have received increasing attention because they can be used as organizational templates in crystallization, metallization, and mineralization (Schnur, 1993) and as drug delivery vehicles (Goldstein et al., 1997). Several types of phospholipids can be induced to form stable tubular structures, such as the helical tubular liposome (a few μm in diameter and 100 μm in length) induced by the Ca2+-mediated inter-membrane interaction in mixtures of cardiolipin and dimyristoylphosphatidylcholine (Lin et al., 1982), the crystal cylindrical structures of Na+-bound dimyristoylphosphatidylglycerol on annealing of a gel phase temperature (Kodama et al., 1993) and the bilayers, tubules, and cochleate myelinic cylinders formed by monoglycosylated sphingolipid homologs (Goldstein et al., 1997; Kulkarni et al., 1995, 1999) that were found originally as intracellular lipid deposits in Gaucher's and Krebbe's diseases (Yanis and Lee, 1970). However, the lipid assemblies are intrinsically fluid in nature. Consequently, their ordered structures are easily disordered by physical or chemical forces acting to transform to the other structures (Hotani et al., 1999). For example, although giant liposomes prepared from the film of dipalmitoylphosphatidylcholine/cholesterol (1/1) can adopt various vesicular structures containing thin flexible filaments through the several transformation pathways from the biconcave form, they do not maintain well-defined structures (Hotani, 1984).
In biology, protein-lipid complexes are involved in the morphological shaping of stable vesicular structures in cellular membrane systems (Rothman and Wieland, 1996). Extensive membrane tubules (50–80 nm in diameter and up to several μm in length), thought to participate in membrane trafficking, have been observed to form the Golgi complex, the trans Golgi network, and the connections between the Golgi stacks of eukaryotic cells (Weidman et al., 1993; Urrutia et al., 1997; Presley et al., 1998). This stable tubule formation is induced by proteins that interact with lipids. For example, the three proteins localized to both synaptic and Golgi intracellular membrane compartments, which all have amphiphilic α-helical regions in their N-terminals, display a membrane tubulating activity (Farsad et al., 2001). But what is the minimal machinery required to form such membrane tubules? In the previous study, we demonstrated that a de novo-designed 18-mer amphiphilic α-helical peptide, Hel 13-5, transforms spherical liposomes prepared from a Golgi-specific phospholipid mixture into nanotubules resembling those from the Golgi apparatus on the scale and the shape (Lee et al., 2001). Furthermore, we showed that the size and the shape of such nanotubules depend on lipid composition, and peptide properties such as length and the ratio of hydrophobic to hydrophilic amino acids. Our work might define the first simple membrane modeling system that can be used to begin to understand how peptides and proteins shape biomembranes into cellular organelles. Interestingly, Mercier et al. (2002) have shown that the biogenesis of nanotubular networks in Toxoplasma parasitophorous vacuoles induced by parasite protein depends on the amphipathic α-helical regions of the protein.
In the present study, to investigate in greater detail the relationship between the peptide sequence and nanotubular structure formation, we designed and synthesized two sets of peptides based on modifying Hel 13-5: the first with varying chain lengths and the second with substituted amino acids as shown in Fig. 1. Their interaction with phospholipids was examined through circular dichroism (CD) spectroscopy, membrane-clearing experiments, liposome size determinations, and morphological observations by electron microscopy.
FIGURE 1.
Primary structure, helical wheel, and helical net representations of the amphiphilic model peptide, Hel 13-5. Symbol n in Hel 13-5Nle is norleucine.
MATERIALS AND METHODS
9-Fluorenylmethoxy-carbonyl (Fmoc)-(Leu or Lys)-polyethylene glycol-polystyrene (PEG-PS) resin, Fmoc amino acids, activation reagents (HOBT and TBTU), and 2,2,2-trifluoroacetic acid were purchased from Watanabe Chem. Ind., Ltd. (Hiroshima, Japan). Egg yolk phosphatidylcholine (egg PC) was purchased from Avanti Polar Lipids (Alabaster, AL, USA). Brain phosphatidylserine (PS) and egg PG were obtained from Sigma Chemical (St. Louis, MO, USA). All the materials described above were used as received. N-Tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid (TES) buffer (5 mM TES/1 mM EDTA/100 mM NaCl at pH 7.4) was used as a buffer solution.
Peptide synthesis
Peptides were synthesized by Fmoc strategy based on the solid phase technique starting from Fmoc-(Leu or Lys)-PEG-PS resin (0.1 mmol scale) using a Pioneer Peptide Synthesis System (Applied Biosystem, Japan) as described previously (Kiyota et al., 1996). The peptide-bound resins were treated by 2,2,2-trifluoroacetic acid (20 ml) containing m-cresol (0.35 ml), ethandithiol (1.05 ml), and thioanisole (2.3 ml) for 1.5 h at room temperature. The crude peptides obtained were applied on Sephadex G-25 (25 mm × 130 cm). Then peptides, L9GHel 13-5, Hel 13-5Nle, Hel 13-5R, E3Hel 13-5, and Hel 8-4, were purified by a preparative C18 reverse phase (RP)-HPLC. Hels 13-5V, 10-4, 12-4, 14-6, and 15-7 were purified by a C8 RP-HPLC column, and Hel 17-7 was purified by a C4 RP-HPLC column. Peptide purities were confirmed by analytical RP-HPLC and MALDI-TOF mass spectroscopy (Voyager-DE STR, PerSeptive Biosystems, Framingham, MA, USA).
The stock solutions of model peptides were prepared as follows: the peptide powders were dampened with only a little 100% acetic acid or trifluoroethanol, then diluted in TES buffer and filtered through the membrane with a pore size of 0.45 μm (Millex-HV, Millipore, MA, USA). The peptide concentrations in the stock solution were determined from UV-absorbance of Trp at 280 nm (ɛ = 5500) in TES buffer containing 6M Gu·HCl.
CD spectrum measurement
CD spectra were measured by a JASCO J-600 spectrometer (JASCO Corporation, Tokyo, Japan) with a personal computer (NEC PC-9801) using a quartz cell with a 1-mm path length. Liposomes were prepared by the extrusion method as described later. Peptide and lipid concentrations were kept at 20 μM and 100 μM, respectively.
Turbidity measurement
Lipid clearing experiments were performed as reported by Mclean et al. (1991). Lipids in chloroform placed in a round-bottom flask were dried through a stream of N2 gas. The residual lipid films were further dried overnight in vacuo and then hydrated with the TES buffer by vortexing. The turbid liposome solutions obtained were diluted to a concentration of ∼100 μM in the same buffer. The peptide solutions were then added to the liposome solution to obtain a given mole ratio of the peptide to lipid and incubated at 25°C. The absorbance of the sample solution was recorded at 400 nm using a JASCO spectrometer (JASCO Corporation, Tokyo, Japan) after vigorous vortexing.
Liposome-size determination
Unilamellar liposomes in a controlled size distribution of ∼80–90 nm in diameter were prepared by using the extrusion method (Mayer et al., 1986). The liposomes, made by ultrasonic irradiation in the cuphorn of a Branson Model 185 sonifier at room temperature, were extruded through two stacked polycarbonate filters with 100-nm and 200-nm pore sizes by applying nitrogen pressure, and then collected and reextruded into an Extruder (Lipex Biomembrane Inc., Vancouver, Canada). The liposome solution (100 μM) was added to the peptide solution of appropriate concentrations. The size of liposomes were measured by the quasi-elastic-light scattering method using a NICOMP Submicron Particle Sizer, Model 370, version 1.46 (Santa Barbara, CA, USA) with an argon laser (λ = 488 nm) of a maximum power of 75 mW at 25°C (Minami et al., 1996).
Electron microscopy
For whole-amount observation, liposome solutions were prepared by the extrusion method described above. The lipid and peptide concentrations were 500 μM and 100 μM, respectively (lipid/peptide = 5/1). All the peptides were incubated for ∼24 h with liposome before the negative staining. The sample solutions were placed on carbon-coated grids and stained with 5% phosphotungstic acid adjusted to pH 7.4, and observed by Hitachi 7100 transmission electron microscope (Hitachi, Japan).
For thin sectioning, the peptide pellets obtained by a 3000 RPM centrifugation step were fixed with 2% (w/v) paraformaldehyde and 0.2% (v/v) glutaraldehyde, in 0.15 M sodium cacodylate buffer (CB), pH 7.4, for 30 min at room temperature. Pellets were rinsed gently three times in 0.1 M CB for 1 h. Osmication was performed in 2% (w/v) osmium tetroxide in 0.1 M CB for 30 min at RT. Pellets were rinsed gently three times in 0.1 M CB. Filtered solution of 0.5% tannic acid in 0.1 M CB was applied for 10 min at room temperature. Pellets were washed immediately in 1% (w/v) sodium sulfate in 0.1 M CB (1, 2, 4, 8 min) and dehydrated in 50% (v/v) ethanol/water for 5 min. A 5% (w/v) uranyl acetate in 50% EtOH for 30 min at RT was applied. Pellets were dehydrated in 70%, 80%, 90%, 95%, 100%, and anhydrous EtOH (×2) for 5 min each and then passed through propylene oxide and 1:1 propylene oxide:Epon 812 for 10 min. Epon 812 mixture was prepared according to the manufacturer's instructions (Electron Microscopy Sciences, Fort Washington, PA, USA). Pellets were embedded in the BEEM capsules. Silver-gold thin sections (60–90 nm) were collected on uncoated (200 mesh thin-bar) copper grids. Grids were counterstained with uranyl acetate and lead citrate, rinsed well in H2O, and dried. Sections were examined at 80 kV in Tecnai 12 TEM (Phillips FEI, Eindhoven, Netherlands); images were collected using BioScan Camera (Gatan, Pleasanton, CA, USA).
RESULTS
Design and synthesis of model peptides
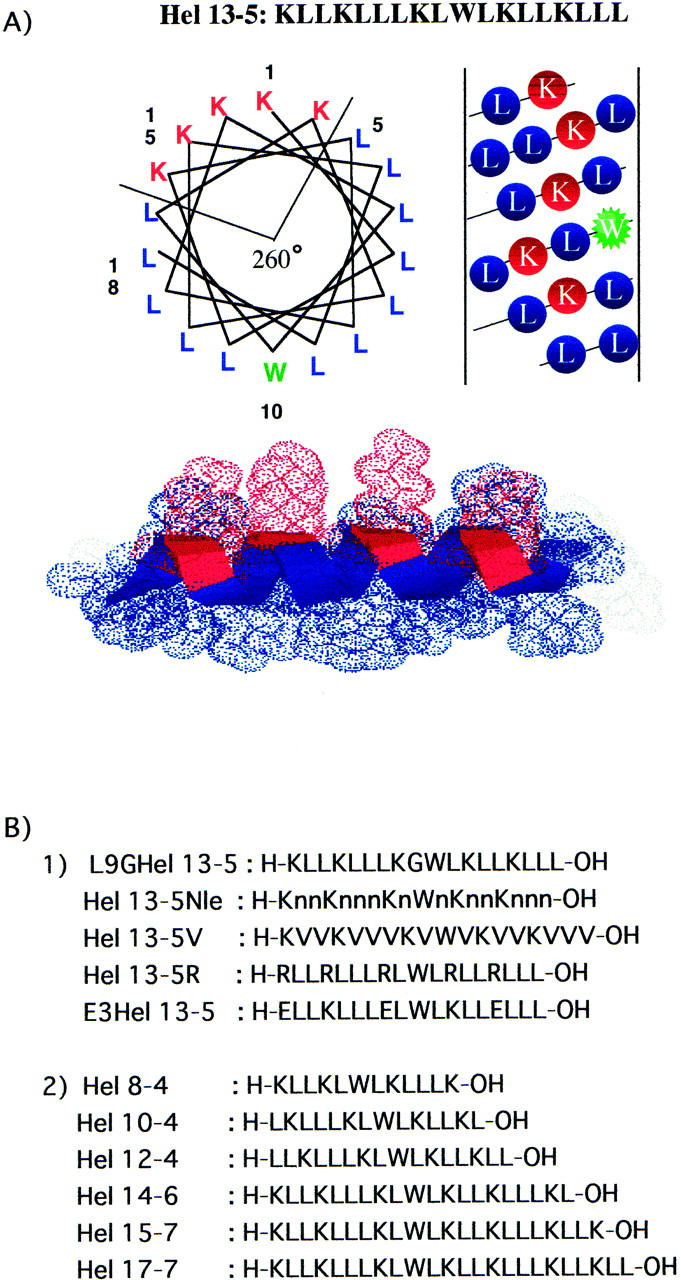
The amphiphilic α-helical peptide, Hel 13-5, consists of 13 hydrophobic residues (12 leucines and one tryptophan) and five hydrophilic residues (five lysines) (Fig. 1 A). When it adopts an ideal α-helical structure, it has an amphiphilic structure with a 260° hydrophobic sector region as shown in helical wheel representation. To investigate the relationship between the peptide sequence or chain length and fibril structure formation, we designed and synthesized two sets of Hel 13-5 related peptides as shown in Fig. 1 B-1. All of these peptides have the same 260° hydrophobic sector region as Hel 13-5. First, to look at the role of hydrophobic residues in amphiphilic α-helical structures, we created Hel 13-5V and Hel 13-5Nle (Nle: norleucine) where all Leu residues are replaced by Val and Nle, respectively, and L9GHel 13-5 where one Leu is replaced by a helix-breaker amino acid, Gly. Second, to investigate the role of hydrophilic residues, we synthesized two peptides, Hel 13-5R, with all Lys residues replaced by Arg, and E3Hel 13-5, with three Lys residues replaced by Glu.
Third, to investigate the effect of chain length, six peptides with residues of even number from 12 to 24 (Hel 8-4 to Hel 17-7) were made (Fig. 1 B-2). For the peptides shorter than the residues of Hel 13-5, each one residue was successively omitted from both N- and C-terminals. Although the 260° hydrophobic sector was retained in these peptides, their ratios of hydrophobic and hydrophilic amino acid residues differed slightly from Hel 13-5.
CD study
To investigate the conformational properties of these peptides, we measured their CD spectra in various media (Fig. 2). In TES buffer solution (Fig. 2 A), L9GHel 13-5, and Hels 13-5R, 13-5, 14-6, 15-7, and17-7, adopted a typical α-helical spectral pattern with a negative ellipticity at 206 and 222 nm and a positive CD band below 200 nm, whereas Hels 8-4, 10-4, 13-5Nle, and 13-5V showed β-structure-like CD bands with a minimum band ∼216 nm. Interestingly, Hel 12-4 adopted a mixture of α-helix and β-structure, indicating that 16 residues might be an intermediate state of conformational change of α-helix to β-structure.
FIGURE 2.
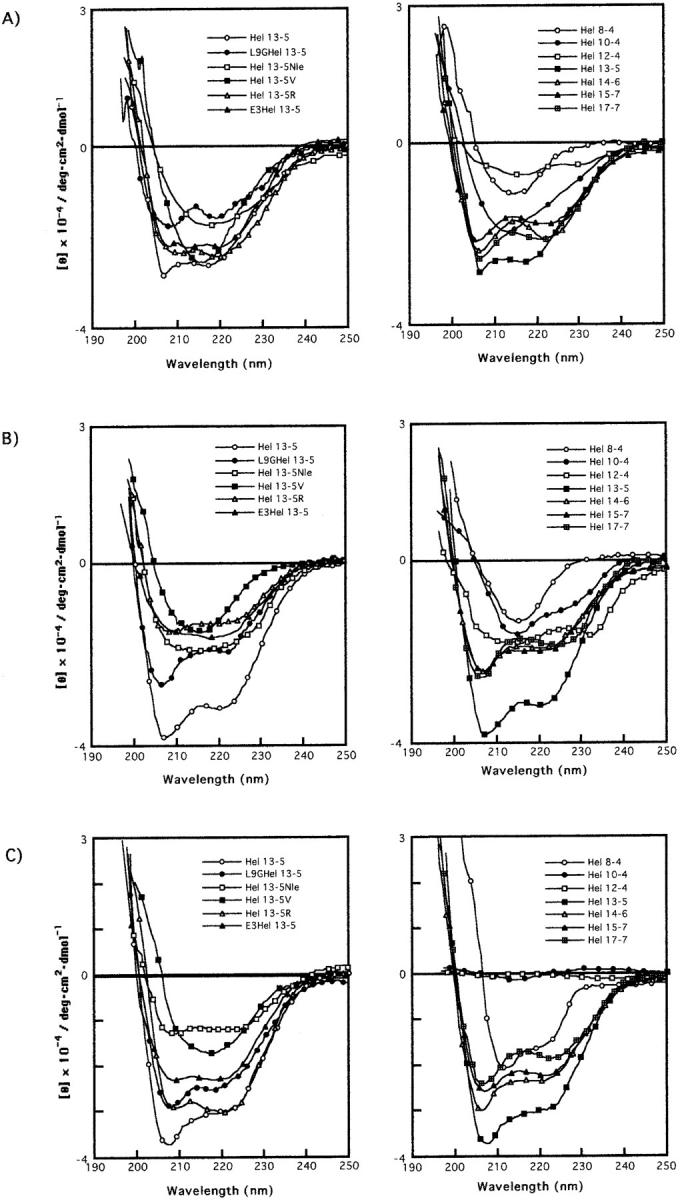
CD spectra of Hel 13-5 and its related peptides in buffer (A), in the presence of egg PC (B) and egg PC and egg PG (3/1)(C). Peptide and lipid concentrations are 20 μM and 100 μM, respectively.
In small unilamellar egg PC liposomes, all the peptides, except for Hel 13-5Nle and E3Hel 13-5, displayed similar α-helical CD shapes ∼200–250 nm as those in buffer solutions, although the intensities of the troughs are different (Fig. 2 B). Hel 13-5Nle and E3Hel 13-5 have a broad band at 205–225 nm, indicating a mixture of α-helix and β-structures.
As shown in Fig. 2 C, all the peptides in the presence of acidic liposomes (egg PC/egg PG = 3/1), except for Hels 13-5V, 10-4, and 12-4, showed α-helical structures, although the helical contents are different. It is noted that Hel 13-5V adopted a β-structural pattern. Hels 10-4 and 12-4 did not show any secondary structural patterns in acidic liposomes.
Measurements of turbidity and mean diameter of liposome
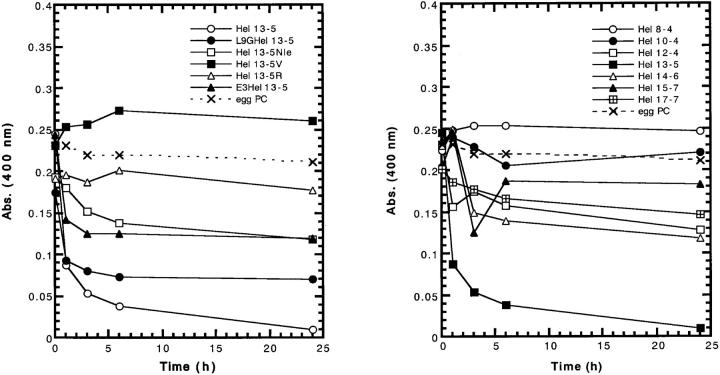
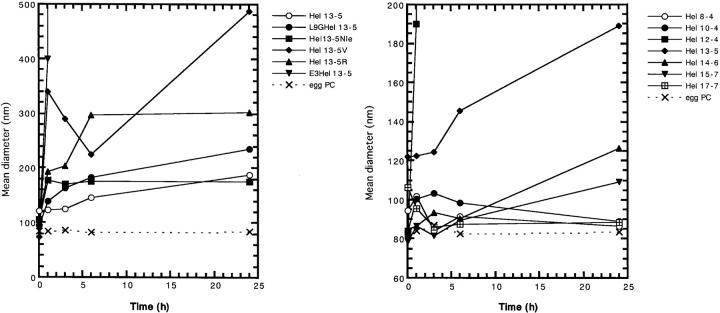
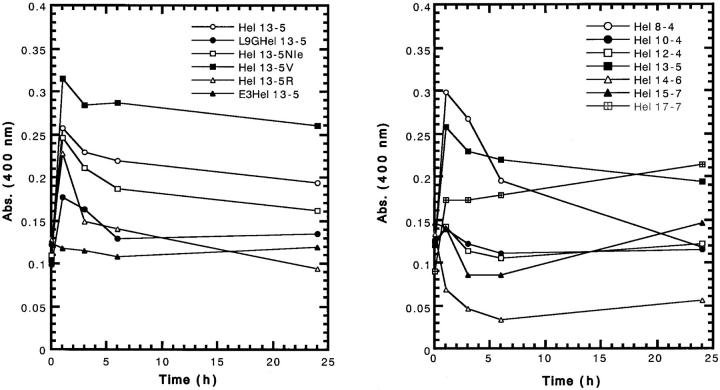
In the previous study, we demonstrated that the fibril structure formation by lipid-peptide complexes leads to a decrease in turbidity of neutral lipid (egg PC) suspensions, and that this accompanies an increase in the mean diameter of the complex, when measured by quasi-elastic-light scattering and analyzed by conventional analysis procedure (Kitamura et al., 1999). Therefore, the time course of turbidity and mean diameter of liposome were measured to investigate any tubular structure formation by the peptides. In the presence of neutral liposomes (100 μM), at the peptide concentration of 20 μM (lipid-peptide ratios = 5/1), the absorbance of L9GHel 13-5, E3Hel 13-5, Hel 12-4, and Hel 14-6 decreased sharply within 1 h and then reached a plateau as shown in Fig. 3 A. This indicates that vesicles become smaller in size than those of pure lipids in the absence of peptides. However the decreases are smaller than those found for Hel 13-5. Gradual decreases in turbidity were observed for Hel 13-5Nle, Hel 13-5R, Hel 17-7, and Hel 10-4. On the other hand, a slight increase in turbidity was observed for Hel 13-5V and Hel 8-4, suggesting that the liposomes were aggregated or fused. Interestingly, for Hel 14-6 and Hel 15-7, their turbidity increased in the early stages and then decreased later, and in the case of Hel 15-7, increased again.
FIGURE 3.
Turbid-liposome clearing ability (upper) and change in mean diameter (lower) of model peptides as a function of time in egg PC liposomes. Peptide and lipid concentrations are 20 μM and 100 μM, respectively.
The mean diameter of neutral liposomes treated by Hel 13-5 analogs also increased gradually to a similar extent for L9GHel 13-5, Hel 13-5R, and Hel13-5Nle as Hel 13-5, as shown in Fig. 3 B. Interestingly, E3Hel 13-5 and Hel 12-4 showed a drastic increase in the mean diameter. These results are consistent with electron micrographs showing that the former peptides formed fiber structure, but that the latter peptides adopted a large aggregated or liposome fused structure, as described below. Hel 13-5V, which adopted a β-structure as determined by CD, showed an increase in the liposome size during the first h, but then decreased in size over the next 6 h, and after that increased in size again. The other peptides with different chain length showed few or no changes in size with time.
In the presence of acidic liposomes, the turbidity for Hel 17-7 slowly increased. On the other hand, a decrease was observed for Hel 14-6 and Hel 15-7, but no change took place for E3Hel 13-5 (Fig. 4). For the other peptides, the turbidity rapidly increased within 1 h, and beyond a maximum, decreased slowly, although the intensities were different. Unfortunately, we could not perform the measurement of size (the mean diameter), because all the acidic liposomes increased in the size beyond the measuring scale (more than 1 μm) of the machine (NICOMP Submicron Particle Sizer), when mixed with any of the peptides.
FIGURE 4.
Turbid-liposome clearing ability of model peptides as a function of time in egg PC/egg PG (3/1) liposomes. Peptide and lipid concentrations are 20 μM and 100 μM, respectively.
Electron microscopy
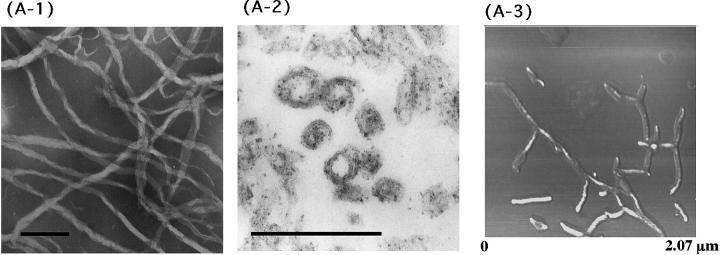
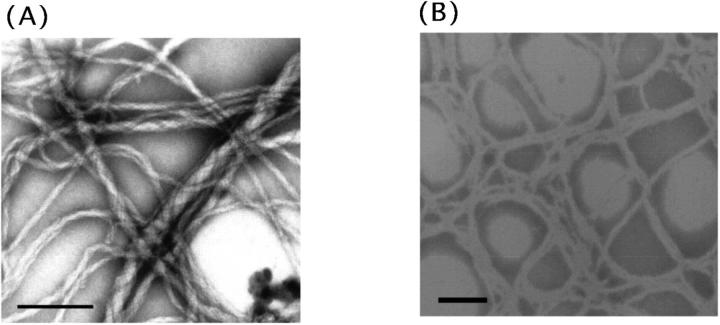
To investigate whether the present peptides adopt a tubular structure like that of Hel 13-5, a transmission electron microscopy (TEM) study was carried out. Egg PC liposomes (500 μM) prepared by the extrusion method in the presence of EDTA were visualized through an electron microscope under negative stain and thin section methods after the addition of peptide (100 μM). The mean diameter of neutral or acidic liposome was ∼80–90 nm. We found that Hel 13-5 transforms egg PC phospholipids into helical fibril structures 25∼50 nm in diameter and more than a few μm in length as shown previously (Fig. 5 A-1) (Kitamura et al., 1999). We also observed double helical structures formed by two fibers. After fixing with 3% glutaraldehyde, thin sections of the Hel 13-5 and egg PC phospholipid mixtures revealed circular- and sheath-like structures indicative of the tubular structure (Fig. 5 A-2). The tubules were ∼50 nm in diameter and 5 nm in thickness corresponding to unilamellar. Some of the tubules adopted more complex structures made from two or three tubules, forming double or triple helical arrangements. Images taken with atomic force microscopy (AFM) also showed left-sided, twisted-helical tubules (Fig. 5 A-3).
FIGURE 5.
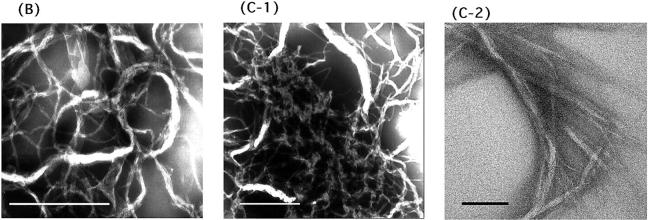
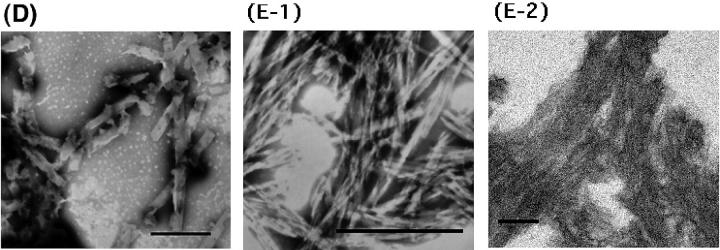
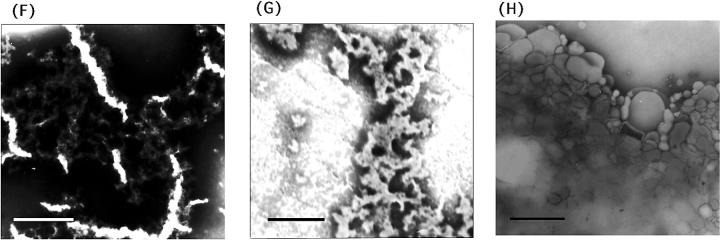
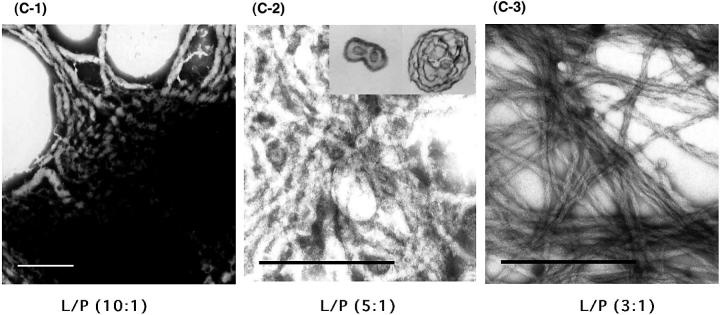
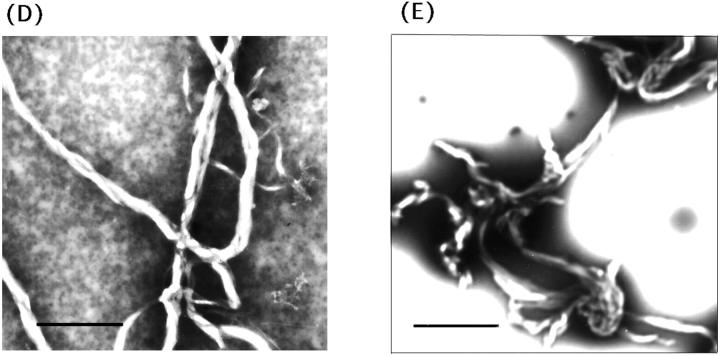
TEM and AFM images by a mixture of Hel13-5 or its relating peptides and egg PC. Hel 13-5: negatively-stained TEM (A-1); thin sections after fixing with 3% glutaraldehyde (A-2); and AFM image on mica (A-3). L9GHel 13-5: negatively-stained TEM (B). Hel 13-5R: negatively-stained TEM (C-1); and thin sectioning (C-2). Hel 13-5Nle: negatively-stained TEM (D). E3Hel 13-5: negatively-stained TEM (E-1); and thin sectioning (E-2). F–H are negatively-stained TEM images of Hel 14-6, Hel 15-7, and Hel 12-4, respectively. Bars are 200 nm in A, and 500 nm in others.
As shown in Fig. 5, B and C, in neutral liposome, L9GHel 13-5 and Hel 13-5R adopted twisted ribbon-like fibers, which were longer than a few μm. They also formed double or more intertwining structures. Interestingly, despite a relatively straight fibril structure for Hel 13-5, the fibril structures of L9GHel 13-5 were more bended. The TEM image of thin sectioning for Hel 13-5 R showed a tubular structure ∼25 nm in diameter and 5 nm in bilayer thickness (Fig. 5 C-2).
Hel 13-5Nle (Fig. 5 D) was found to form short rod-like structures that were much shorter (∼25–50 nm in diameter and 0.5–1 μm in length) than the structure formed by Hel 13-5. Interestingly, E3Hel 13-5 (Fig. 5 E-1) also created very short fibril structures (∼25–50 nm in diameter and 300 nm in length) that were similar to those of Hel 13-5Nle, but the structures of the former aggregated more than those of the latter. The thin-section TEM images showed that the tubular structures of E3Hel 13-5 (Fig. 5 E-2) are layered just as the Golgi apparatus tubules, but that those of Hel 13-5Nle are not. Hel 13-5Nle and E3Hel 13-5 created a mixture of α-helix and β-structures in egg PC liposome, indicating that the fiber shapes may also depend on their secondary structure.
For Hel 13-5V, spherical liposomes of more than 200 nm in diameter, but no fibril structures were observed (data not shown), suggesting that the β-structural peptide makes small liposomes fuse. Taken together, these facts have the following implications on the relationship between the nature of the peptide sequence and its ability to form tubules: the tubular structure can be retained when the hydrophobic isobutyl groups of the Leu residues are interchanged with n-buthyl groups but not with isopropyl groups, and can also be retained when the charged Lys residues in the hydrophilic regions of amphiphilic peptides are replaced by Arg or Glu.
Regarding the different chain length model peptides, short twisted fibril structures (∼1 μm in length) as well as intertwined fibrils were formed for Hel 14-6 (Fig. 5 F). Aggregates formed from short rod-type structures were observed for Hel 15-7 (Fig. 5 G). No fibril structure was observed for Hels 8-4, 10-4, 12-4, and 17-7. Interestingly, for Hel 12-4, larger spherical liposomes were observed, suggesting the fusion of the original liposomes (Fig. 5 H). These results tell us that the 18 chain residues in Hel 13-5 are extremely critical determinants of long fibril structure formation, at least with neutral liposomes.
In neutral/acidic phospholipid mixtures (PC/PG and PC/PS; 3/1), left-sided helical tubular fibers similar to those seen with pure PC liposomes were also found for Hel 13-5 (Fig. 6, A and B). Thus, to investigate how the charge interactions between lipid head groups and the hydrophilic residues of peptides and also how the hydrophobic interactions of the peptides and lipids contribute to the fiber formation, the lipid-peptide ratio (L/P) for the mixture of PC/PG (3:1) was varied (Fig. 6 C). For L/P = 10/1 (equivalent charge ratio), short elliptical fused-liposomes aggregated to form short fibers (50–100 nm in thickness and less than 1 μm in length; Fig. 6 C-1). For L/P = 5/1 (in 2× higher negative charge) various sized fibers (25–80 nm in thickness and more than 1 μm in length) were formed; the elliptical liposomes seen for L/P = 10/1 grew into fused fiber structures with one to multi circular tubular structures (Fig. 6, C-2 and inset). Finally, for L/P = 3/1, much longer fiber structures with the same diameter (25 nm in thickness) were formed (Fig. 6 C-3). These results suggest that the mechanism of microtubule formation did not involve the charge interaction, but the immersion of the hydrophobic part of the peptides into liposomes—initially forming elliptic-like structures by the fusion of small liposomes, and then, with increasing peptide concentrations, forming more ordered fiber structures. It should be noted that, as shown in Fig. 6, D and E, fibril structures were observed for L9GHel 13-5 and Hel 13-5R in acidic liposomes; however, all the other peptides did not form fibril structures in acidic liposomes.
FIGURE 6.
TEM images by a mixture of Hel13-5 or its relating peptides and acidic phospholipids. Hel 13-5 in PC/PG (3/1), (A), and in PC/PS (3/1), (B). Different lipid/peptide ratio (L/P) of Hel 13-5 in PC/PG (3/1): L/P (10/1), (C-1); L/P (5/1), (C-2);and L/P (3/1), (C-3). L9GHel 13-5 in PC/PG (3/1), (D). Hel 13-5R in PC/PG (3/1), (E). Bars are 500 nm in A, C, and D; 200 nm in B; and 667 nm in E.
DISCUSSION
We have previously discussed that the fibril structures formed by lipid-peptide complexes leads to a decrease in turbidity of neutral lipid (egg PC) suspensions, and that it accompanies an increase in mean diameter of the complex as measured by quasi-elastic-light scattering (Kitamura et al., 1999). The turbidity depends on the difference in the refractive indices between the scattering entity and the medium; the larger the difference, the more turbid the solution becomes. It is likely that the lipid and peptide molecules are packed more loosely in the fibril assembly than in the liposomal assembly; in this case, the difference in the refractive indices between the molecular assembly and aqueous phase would be smaller for the fibril structure than the liposomal structure. The addition of Hel 13-5 to the neutral liposome solution results in the decrease in turbidity accompanied by the transformation from liposome to fibril structure. In dynamic light scattering, on the other hand, the “size” of particles in solution is evaluated based on the translational diffusion coefficient of one particle, which is reflected in the fluctuation of scattered light intensity. The diffusion rate of the solutes with fibril structure should be quite low. Thus, the size of the molecular assembly with fibril structure would become apparently very large when derived from dynamic light scattering using a conventional analysis procedure. Along the same lines, we consider here how the secondary structures of peptides and the change in turbidity or the liposome size change do relate to the fibril structure formation. The experimental results will be discussed in terms of the CD, turbidity, and liposome size experiments, as they relate to the electron microscopy observations.
Hel 13-5, L9GHel 13-5, and Hels 13-5R, 14-6, 15-7, and 17-7 were found to adopt a mainly α-helical structure at the peptide concentration of 20 μM in egg PC liposomes of 100 μM (Fig. 2 B). All these peptides except for Hel 13-5R decreased the turbidity of hydrated multilamellar vesicles after 24 h incubation (Fig. 3 A). L9GHel 13-5 and Hel 13-5R showed increases in the mean diameter of liposomes as compared with the original liposomes (Fig. 3 B). Actually, on the observation of electron microscopy, tubules formed by the addition of L9GHel 13-5 or Hel 13-5R to egg PC liposome are similar in chain length to those of Hel 13-5. Thus, even if an α-helix breaker amino acid, Gly, is introduced into the hydrophobic region, the peptide that can adopt α-helical structure makes the tubular structure. And the data also showed that the Lys residues of the hydrophilic side of the amphiphilic structure can be replaced with other basic residues. We cannot explain the reason why Hel 13-5R did not decrease the turbidity.
Hel 14-6 also decreased the turbidity, but the mean diameter of liposomes did not change very much. Under electron microscopy, short single helical fibrils were observed for Hel 14-6, indicating that Hel 14-6 has no ability to make the long fibril structures similar to Hel 13-5. In regard to other peptides, Hels 10-4, 15-7, and 17-7, a decrease of turbidity but no increase of liposome size was observed. Hel 15-7 could make a short rod-type structure, but no fibril structure was viewed for those peptides under electron microscopy. These results indicate that even if the peptides take α-helical structure, they cannot always make fibril structure. Clearly, the length of the peptide is a determining factor in tubule creation, and the 18-mer residues in Hel peptides are very critical determinants in the creation of long tubular structures.
Hel 13-5Nle and E3Hel 13-5, which have a broad negative band at 205–225 nm indicating a mixture of α-helix and β-structure (Fig. 2 B), led to a decrease in turbidity. The mean diameter of neutral liposomes, when incubated with E3Hel 13-5, drastically increased within 1 h. Hel 13-5Nle also increased moderately. On the electron micrographs, Hel 13-5Nle and E3Hel 13-5 were observed to form the short tubular structures that are much shorter than the structure formed by Hel 13-5 (Fig. 5, D and E). Although both peptides have a tubular structure as seen in a thin sectioning TEM image, E3Hel 13-5 took an aggregated structure of tubules such as that found with the Golgi's tubular complex (Fig. 5 E-2). A mixture of α-helix and β-structures, which was created in the presence of egg PC liposome, may determine the tubular shapes. Interestingly, when Lys residues of the hydrophilic region in the amphiphilic structure of Hel 13-5 were substituted for Arg, and when partially substituted even for Glu, the fibril structures were observed equally, indicating that the hydrophilic part of the amphiphilic α-helical structure in Hel 13-5 is not critical for the tubular structure formation. The glutamic residues in E3Hel 13-5 may promote the tubule aggregation by the charge interaction between tubules. Hel 12-4, which also showed a mixed CD curve of α-helical and β-structure (Fig. 2 B), had a moderate decrease in the turbidity and a remarkable increase in liposome size within 1 h (Fig. 3, A and B). These results are not incompatible with that of the electron micrograph; no fibril structures, but large fused liposomes were observed (Fig. 5 H). It is unclear why Hel 12-4 could not form a tubular structure. However, Hel 10-4, which takes a mixed CD curve of α-helical and β-structure, did not form such structures—indicating that amphiphilic peptides of less than 18 residues could not transform the lipid to tubules.
It is noted that Hel 13-5V as well as Hel 8-4 adopted β-structure in egg PC liposomes and that Hel 13-5V increased in both turbidity and liposome size, whereas Hel 8-4 did not. However, no tubular structure was seen with electron microscopy for Hel 13-5V and Hel 8-4. Inasmuch as these peptides do not adopt ideal amphiphilic structures when they adopt β-structures, the peptide-lipid interaction is weaker, resulting in no tubule formation.
It is well known in the case of neutral liposomes such as PC that membrane perturbation and fusion by the insertion of peptides into lipid bilayers depend on their hydrophobic interactions. On the other hand, in acidic liposomes such as PG and PS, associations of lipid bilayers should be connected to electrostatic interaction among the polar residues of the peptides and the phospholipid headgroup as well as the hydrophobic interaction (Palozov et al., 1997; Pérez-Méndez et al., 1998). In the present study, the negative-stained TEM study showed that only three peptides, Hel 13-5, L9GHel 13-5, and Hel 13-5R formed fibril structures in the presence of acidic liposomes (egg PC/brain PG = 3/1; Fig. 6). These results suggest that the formation of the fibril structure simultaneously in either neutral or acidic liposomes is very peptide-specific and that the charge interaction of lipid headgroup and the hydrophilic part of peptides is not so important to participate in the fibril structure formation. Considering that the cationic Lys can be replaced with anionic Glu in Hel 13-5 for tubular structure formation (i.e., charge is not so important), the interaction of Hel series peptides and lipids is controlled by hydrophobic interaction between lipid acyl chains and the hydrophobic residues of the peptides.
The exact molecular arrangement of the tubular structures formed by the interaction between Hel 13-5 and phospholipids is unknown. Some two-long alkyl chain amphiphiles can adopt stable tubular structures including twist ribbons, helices, and cochleate cylinders in aqueous media (Schnur, 1993; Goldstein et al., 1997; Kodama et al., 1993; Nakashima et al., 1985; Zastavker et al., 1999). Recent theoretical studies have estimated that intrinsic bending of rectangular bilayer lipid sheets due to chiral packing of molecules in a membrane leads to the formation of tubules (Selinger et al., 1996; Nandi and Bagchi, 1996). The present electron micrograph studies show that all the tubules formed by Hel 13-5 and various phospholipids have twisted helical structures. We have previously shown that the hydrophobic parts of Hel 13-5 are deeply buried into lipid bilayers (Kitamura et al., 1999). It is reasonable, therefore, to hypothesize that the hydrophobic part of amphiphilic Hel 13-5 buried in various phospholipid bilayers helps to bend the rectangular bilayer to make and retain a tubular structure. The immersion of the hydrophobic part of the amphiphilic peptide into lipid bilayers initially induces elliptic-like structures by the fusion of small liposomes and then the elliptic-like structures are transformed into the long fiber structures (Fig. 6 C). However, further study is needed to determine the exact molecular arrangement of peptides in lipid bilayers.
Our present results do provide a starting point for the investigation of how biological systems generate and maintain organelle morphology, or even more interestingly, how dynamic interchanges between different morphologies may occur. In a previous study, we carried out a systematic study using defined lipid mixtures of biological relevance along with defined peptide structures to illustrate and define specific parameters that may determine such morphology (Lee et al., 2001). Our present finding also indicates that tubulovesicular shapes can be controlled by lipid composition and peptide structure. Of course, we do not consider that the present experimental results could directly apply to the interpretation of the complicated tubular formation mechanisms involved in the biological events. However, recent evidences from cell-free systems have shown that dynamin, amphyphisin, and endophilin-1, which have been proposed to participate in the endocytosis of synaptic vesicles, can transform spherical protein-free liposomes composed of a brain lipid extract into narrow tubules (Takei et al., 1998, 1999; Aridor et al., 2001). Although brain extract is not a simple phospholipid model system, the narrow tubules obtained from the system are very similar to ours. An amino acid homolog study of amphyphisin, and of endophilin-1, suggested that the region of lipid binding and tubulation of both proteins needs a sequence of 29 amino acids and its helical wheel plot gives an amphiphilic structure (Farsad et al., 2001). Mercier et al. (2002) also demonstrated that the formation of tubular architecture of an intracellular parasite (Toxoplasma) network requires an amphiphilic α-helical structure composed of 19-mer amino acid residues in the secretary protein, GRA2, from dense granules. Thus, these results and our results suggest that the tubular structures found in intracellular trafficking might be induced and maintained by short amphiphilic α-helical or a mixture of α-helical and β-structural sequences found in membrane-binding surface proteins.
The control of nanotubular structures is important because of their role in various biomedical and biotechnological processes. However, inasmuch as the lipid assemblies are intrinsically fluid, their ordered structures are easily disordered or transformed to the other ones (Hotani et al., 1999). In living bodies, these tubular structures maintain their shapes only if further transformations are prevented by linkage networks of surface membrane proteins. On the other hand, lipid tubules have been studied for over a decade because of their potential application in nanotechnology, such as in metallization, the mineralization-microfabrication process (Schnur, 1993), and as drug delivery vehicles (Goldstein et al., 1997). Our present study of the interaction between the Hel 13-5 and its related peptides and phospholipids shows that lipid-peptide complexes make stable tubular structures with relatively constant diameters in the solution, and that their shapes and sizes can be also controlled by the selection of specific lipid species and peptide sequences. These findings would form part of the groundwork necessary to eventually understand the mechanisms of nanotubular structure formation in intracellular processes, and in the design processes of biotechnological nanotubular materials.
Acknowledgments
This work was supported in part by an operating grant, #12680665, from the Japanese government's Grants-In-Aid for Scientific Research.
References
- Aridor, M., K. N. Fish, S. Bannykh, J. Weissman, T. H. Roberts, J. Lippincott-Schwartz, and W. E. Balch. 2001. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J. Cell Biol. 152:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsad, K., N. Ringstad, K. Takei, S. R. Floyd, K. Rose, and P. De Camilli. 2001. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell Biol. 155:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, A. S., A. N. Lukyanov, P. A. Carlson, P. Yager, and M. H. Gelb. 1997. Formation of high-axial-ratio-microstructures from natural and synthetic sphingolipids. Chem. Phys. Lipid. 88:21–36. [DOI] [PubMed] [Google Scholar]
- Hotani, H., F. Nomura, and Y. Suzuki. 1999. Giant liposomes: from membrane dynamics to cell morphogenesis. Curr. Opin. Colloid Interface Sci. 4:358–368. [Google Scholar]
- Hotani, H. 1984. Transformation pathways of liposomes. J. Mol. Biol. 178:113–120. [DOI] [PubMed] [Google Scholar]
- Kiyota, T., S. Lee, and G. Sugihara. 1996. Design and synthesis of amphiphilic α-helical model peptides with systematically varied hydrophobic-hydrophilic balance and their interaction with lipid- and bio-membranes. Biochemistry. 35:13196–13204. [DOI] [PubMed] [Google Scholar]
- Kitamura, A., T. Kiyota, M. Tomohiro, A. Umeda, S. Lee, and G. Sugihara. 1999. Morphological behavior of acidic and neutral liposomes induced by basic amphiphilic alpha-helical peptides with systematically varied hydrophobic-hydrophilic balance. Biophys. J. 76:1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama, M., T. Miyata, and T. Yokoyama. 1993. Crystalline cylindrical structures of Na(+)-bound dimyristoylphosphatidylglycerol as revealed by microcalorimetry and electron microscopy. Biochim. Biophys. Acta. 1168:243–248. [PubMed] [Google Scholar]
- Kulkarni, V. S., W. H. Anderson, and R. E. Brown. 1995. Bilayer nanotubes and helical ribbons formed by hydrated galactosylceramides: acyl chain and headgroup effects. Biophys. J. 69:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, V. S., J. M. Boggs, and R. E. Brown. 1999. Modulation of nanotube formation by structural modifications of sphingolipids. Biophys. J. 77:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S., T. Furuya, T. Kiyota, N. Takami, K. Murata, Y. Niidome, D. E. Bredesen, H. M. Ellerby, and G. Sugihara. 2001. De novo-designed peptide transforms Golgi-specific lipids into Golgi-like nanotubules. J. Biol. Chem. 276:41224–41228. [DOI] [PubMed] [Google Scholar]
- Lin, K., R. M. Weis, and M. McConnell. 1982. Induction of helical liposomes by Ca2+-mediated intermembrane binding. Nature. 296:164–165. [DOI] [PubMed] [Google Scholar]
- Mayer, L. D., M. J. Hope, and P. S. Cullis. 1986. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochem. Biophys. Acta. 856:161–168. [DOI] [PubMed] [Google Scholar]
- Mclean, L. R., K. A. Hagaman, T. J. Owen, and I. L. Krstenansky. 1991. Minimal peptide length for interaction of amphipathic alpha-helical peptides with phosphatidylcholine liposomes. Biochemistry. 30:31–37. [DOI] [PubMed] [Google Scholar]
- Mercier, C., J. Dubremetz, F. Rauscher, B. Lecordier, L. D. Sibley, and M. R. Cesbron-Delauw. 2002. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite protein. Mol. Biol. Cell. 13:2397–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami, H., T. Inoue, and R. Simozawa. 1996. Beryllium ion can induce the aggregation of phosphatidylcholine vesicles. Langmuir. 12:3574–3579. [Google Scholar]
- Nakashima, N., S. Asakuma, and T. Kunitake. 1985. Optical microscopic study of helical superstructures of chiral bilayer membranes. J. Am. Chem. Soc. 107:509–510. [Google Scholar]
- Nandi, N., and B. Bagchi. 1996. Molecular origin of the intrinsic bending force for helical morphology observed in chiral amphiphilic assemblies: concentration and size dependence. J. Am. Chem. Soc. 118:11208–11216. [Google Scholar]
- Palozov, I. V., A. I. Palozov, E. M. Tytler, G. M. Anantharamaiah, J. P. Segrest, G. A. Woolley, and R. M. Epand. 1997. Role of lipids in the permeabilization of membranes by class L amphipathic helical peptides. Biochemistry. 36:9237–9245. [DOI] [PubMed] [Google Scholar]
- Pérez-Méndez, O., B. Vanloo, A. Decout, M. Goethals, F. Peelman, J. Vandekerckhove, R. Brasseur, and M. Rosseneu. 1998. Contribution of the hydrophobicity gradient of an amphipathic peptide to its mode of association with lipids. Eur. J. Biochem. 256:570–579. [DOI] [PubMed] [Google Scholar]
- Presley, J. F., C. Smith, K. Hirschberg, C. Miller, N. B. Cole, K. J. M. Zaal, and J. Lippincott-Schwartz. 1998. Golgi membrane dynamics. Mol. Biol. Cell. 9:1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J. E., and F. T. Wieland. 1996. Protein sorting by transport vesicles. Science. 272:227–234. [DOI] [PubMed] [Google Scholar]
- Schnur, J. M. 1993. Lipid tubules: a paradigm for molecularly engineered structure. Science. 262:1669–1676. [DOI] [PubMed] [Google Scholar]
- Selinger, J. V., F. C. MacKintosh, and J. M. Schnur. 1996. Theory of cylindrical tubules and helical ribbons of chiral lipid membranes. Phys. Rev. E. 53:3804–3818. [DOI] [PubMed] [Google Scholar]
- Takei, K., V. Haucke, V. Slepnev, K. Farsad, M. Salazar, H. Chen, and P. De Camilli. 1998. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 94:131–141. [DOI] [PubMed] [Google Scholar]
- Takei, K., V. I. Slepnev, V. Haucke, and P. De Camilli. 1999. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1:33–39. [DOI] [PubMed] [Google Scholar]
- Urrutia, R., J. R. Henley, T. Cook, and M. A. McNiven. 1997. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc. Natl. Acad. Sci. USA. 94:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidman, P., R. Roth, and J. Heuser. 1993. Golgi membrane dynamics imaged by freeze-etch electron microscopy: views of different membrane coatings involved in tubulation versus vesiculation. Cell. 5:123–133. [PubMed] [Google Scholar]
- Yanis, E. R., and R. E. Lee. 1970. Tubules of globoid leukodystrophy: a right-hand helix. Science. 169:64–66. [DOI] [PubMed] [Google Scholar]
- Zastavker, Y. V., N. Asherie, A. Lomakin, J. Pande, J. M. Donovan, J. M. Schnur, and G. B. Benedek. 1999. Self-assembly of helical ribbons. Proc. Natl. Acad. Sci. USA. 96:7883–7887. [DOI] [PMC free article] [PubMed] [Google Scholar]