FIGURE 4.
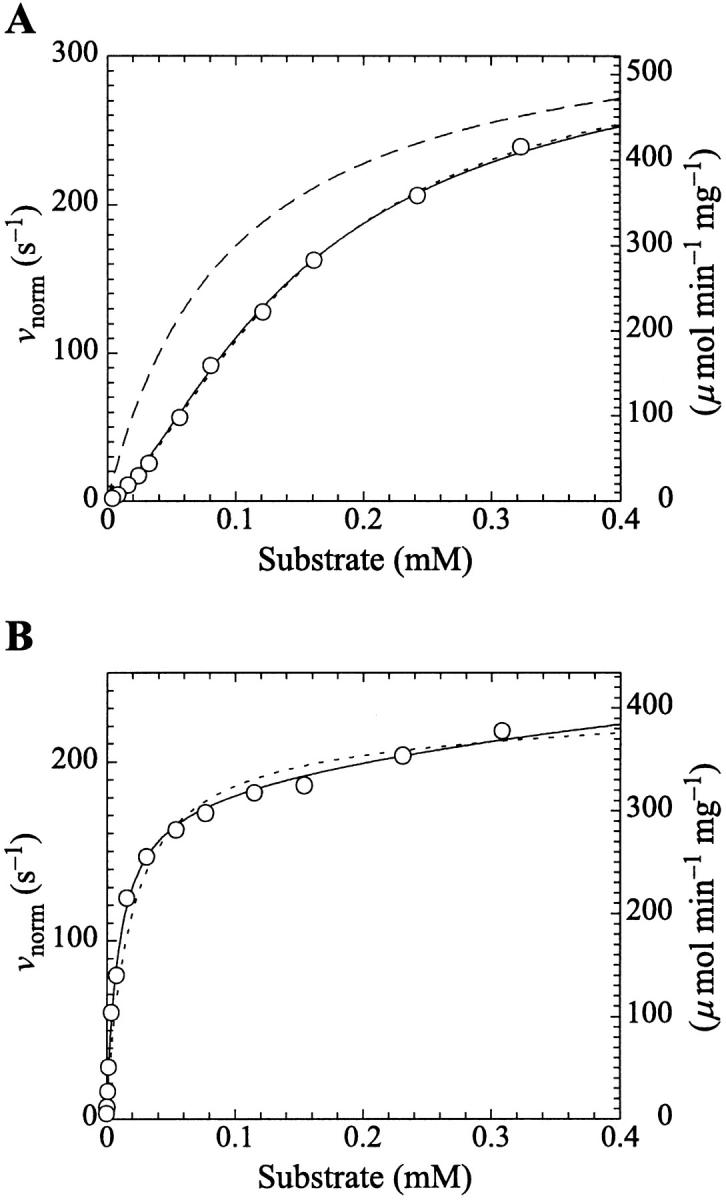
PI-PLC kinetics with butyl-FLIP substrate. (A) Cleavage of butyl-FLIP without activator gives sigmoidal kinetics. The dotted line is a plot of the Hill equation, with n = 1.5. The solid line was calculated for the two-site model using Eq. 2. The reference curve (dashed line) represents the hypothetical case where the only productive complex is SES. (B) Cleavage of butyl-FLIP in the presence of a fixed concentration of activator lipid, 1.07 mM diC6PC, yields approximately hyperbolic kinetics. The dotted line was calculated using Eq. 5 for the general two-site model under the stringent requirement that the input parameters KS, α′, 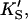 and β′ be identical to those in panel A where no activator is present, and only the two parameters—α and β describing the activator-enzyme complex, and kp—were allowed to vary. The solid line was also calculated using Eq. 5, but relaxing the restriction on the variation of other parameters. Parameter values are given in Table 1. The initial rates are plotted using two common conventions in enzymology: μmol of product per second per μmol enzyme (abbreviated s−1) and μmol of product per minute per mg enzyme (abbreviated μmol min−1 mg−1). The same conventions are used in the following figures. To convert between these two scales, multiply initial rates in μmol min−1 mg−1 by 34.53/60 to obtain rates in s−1. The factor of 34.53 is the molecular weight of B. cereus PI-PLC in kDa, and the divisor is the conversion from minutes to seconds. The PI-PLC concentration was 12.9 pM in both experiments.
and β′ be identical to those in panel A where no activator is present, and only the two parameters—α and β describing the activator-enzyme complex, and kp—were allowed to vary. The solid line was also calculated using Eq. 5, but relaxing the restriction on the variation of other parameters. Parameter values are given in Table 1. The initial rates are plotted using two common conventions in enzymology: μmol of product per second per μmol enzyme (abbreviated s−1) and μmol of product per minute per mg enzyme (abbreviated μmol min−1 mg−1). The same conventions are used in the following figures. To convert between these two scales, multiply initial rates in μmol min−1 mg−1 by 34.53/60 to obtain rates in s−1. The factor of 34.53 is the molecular weight of B. cereus PI-PLC in kDa, and the divisor is the conversion from minutes to seconds. The PI-PLC concentration was 12.9 pM in both experiments.
