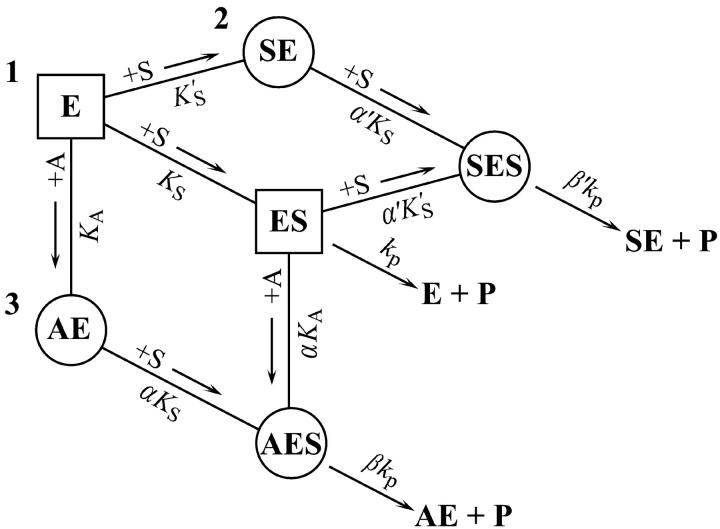
FIGURE 7.
Summary of the two-site model. This model is a combination of the two schemes of Fig. 2. Three branches lead to product, P. The activator site can be occupied by diC6PC (branch 3) or by butyl-FLIP (branch 2), but not by methyl-FLIP, which does not bind significantly to the activator site under the experimental conditions. Any one branch by itself would yield a hyperbolic curve representative of simple Michaelis-Menten kinetics. Sigmoidal kinetics are observed for butyl-FLIP because the binding of S at the subsite to form SE increases the affinity of the active site for S, and the enzyme switches from ES (branch 1) to SES (branch 2) with increasing [S]. The squares and circles indicate two different conformations of residues in the active site/interfacial binding region. Abbreviations are the same as in Fig. 2.