FIGURE 6.
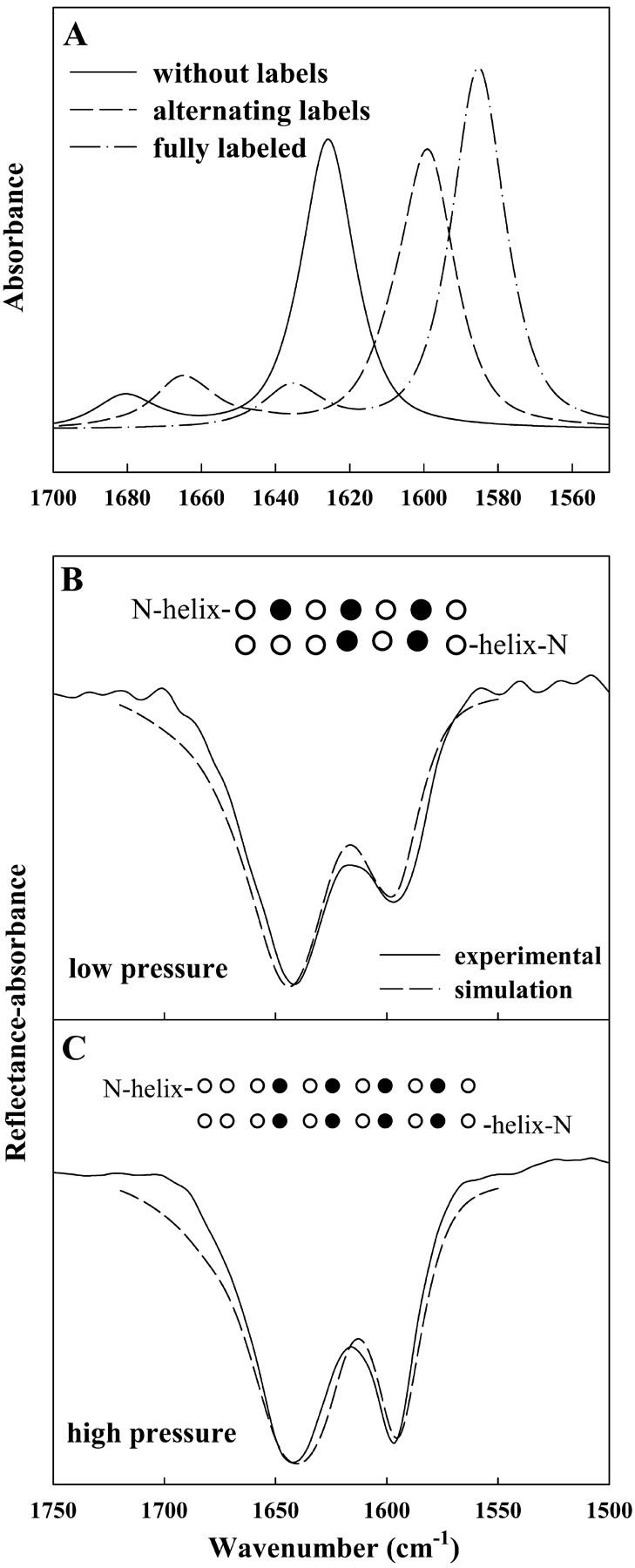
Calculated IR spectra of the Amide I region using a transition dipole coupling method as described by Brauner et al. (2000). (A) Simulations of three peptides, 12 residues in each strand, arranged in an antiparallel β-sheet geometry: (—) all unlabeled residues, (– –) alternating 13C=O/12C=O, and (– · –) all 13C=O labeled residues. (B) Experimental spectrum of *SP-B9–36 acquired at a pressure of 4 mN/m overlaid with an inverted simulated spectrum of two identical peptides with their C-termini in an antiparallel β-sheet geometry. Each peptide consists of 14 residues in an α-helical geometry coupled to an antiparallel β-sheet strand containing seven residues with 13C=O labels as noted (•) in inset. (C) Experimental spectrum acquired at a pressure of 29 mN/m and simulated spectrum similar to B, except that the β-strand portion of each peptide consists of 11 residues, with the labeling differing as noted in the inset. The β-sheet components in the simulated spectra in B and C were multiplied by 1.5 before being added to the helical component.
