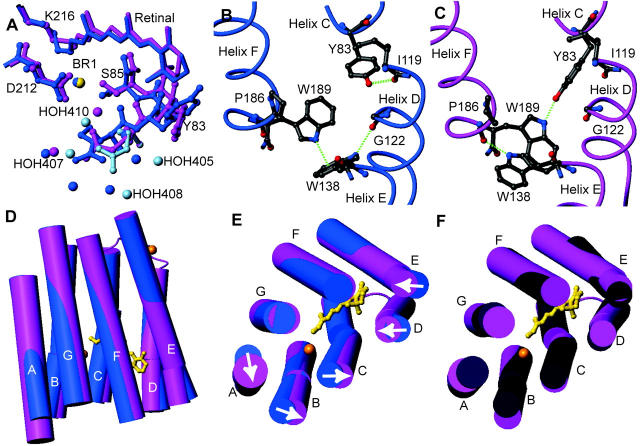
FIGURE 2.
Conformational changes that occur in bR(D85S) upon binding bromide ions. The figures were generated and models were aligned using the program MOLMOL (Koradi et al., 1996). (A) Changes in rotamer conformation of the R82 side chain. The dark-blue model is the halide-free structure, whereas the pink and light-blue models represent the presumed functional and nonfunctional states of the halide-bound structure, respectively. The little spheres represent water molecules and the bromide-ion substrate. (B) Amino acid side-chain positions in the blue, halide-free state and the helix-helix contacts for selected residues on the extracellular side of the protein. Hydrogen bonds are shown with dashed green lines. (C) The same residues from panel B are shown in the purple, halide-bound state. Note that a wild-type ground-state like connection between Y83 and W189 is established upon halide binding, thus creating a direct interaction between helices C and F. (D) A side view of the protein with cylinder representations of helices. The retinal chromophore is drawn in a gold ball-and-stick representation to help orient the molecule for the reader, and the inorganic ions are drawn as gold spheres. The halide-free model is painted blue whereas the halide-bound representation is painted pink. Note that helix A appears longer, due to the addition of well-ordered residues 4–8. (E) Substrate binding induces a repacking of helices on the extracellular side of the protein. Arrows indicate the direction of helical movements due to anion binding in bR(D85S). (F) The comparison between the positions of helices in bR(D85S), bR(D85S) halide-bound (pink), and ground state of wild-type bR (dark purple).