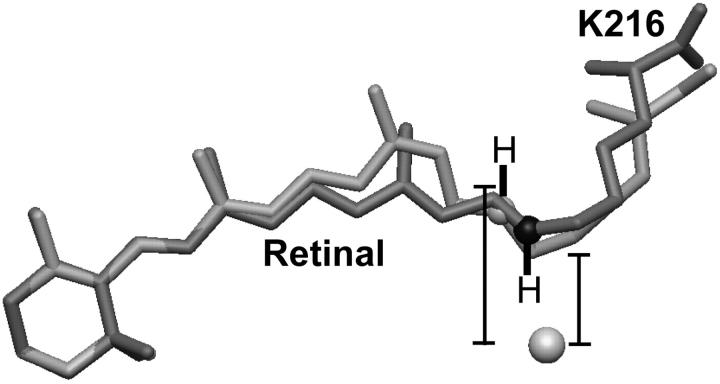
FIGURE 5.
Isomerization of the retinal moves the Schiff-base counter ion away from the halide substrate. The bromide ion is shown as a sphere, whereas the model of the all-trans retinal group bound covalently to K216 is shaded dark gray for the bromide-bound form of bR(D85S) and light gray for the model of the D96N late-M intermediate. In addition, the K216-NZ atoms of the D96N late-M intermediate and bromide-bound bR(D85S) models are shown as light- and dark-gray spheres, respectively. The hydrogen atoms and their bonds to K216-NZ were drawn by hand. Distances were measured from the bromide ion to the midpoint of the Schiff-base N–H bond. The models shown here were aligned by minimizing the RMSD of the C1, C2, C3, C4, C5, and C6 atoms of the retinal ring between the two models. This procedure, in contrast to aligning the models by minimizing the RMSD between selected CA atoms, seems to minimize the difference in position between the K216-NZ atoms, thereby minimizing the apparent energetic effect of the charge displacement. The actual charge displacement may thus be larger than that which is reported in the text.