FIGURE 6.
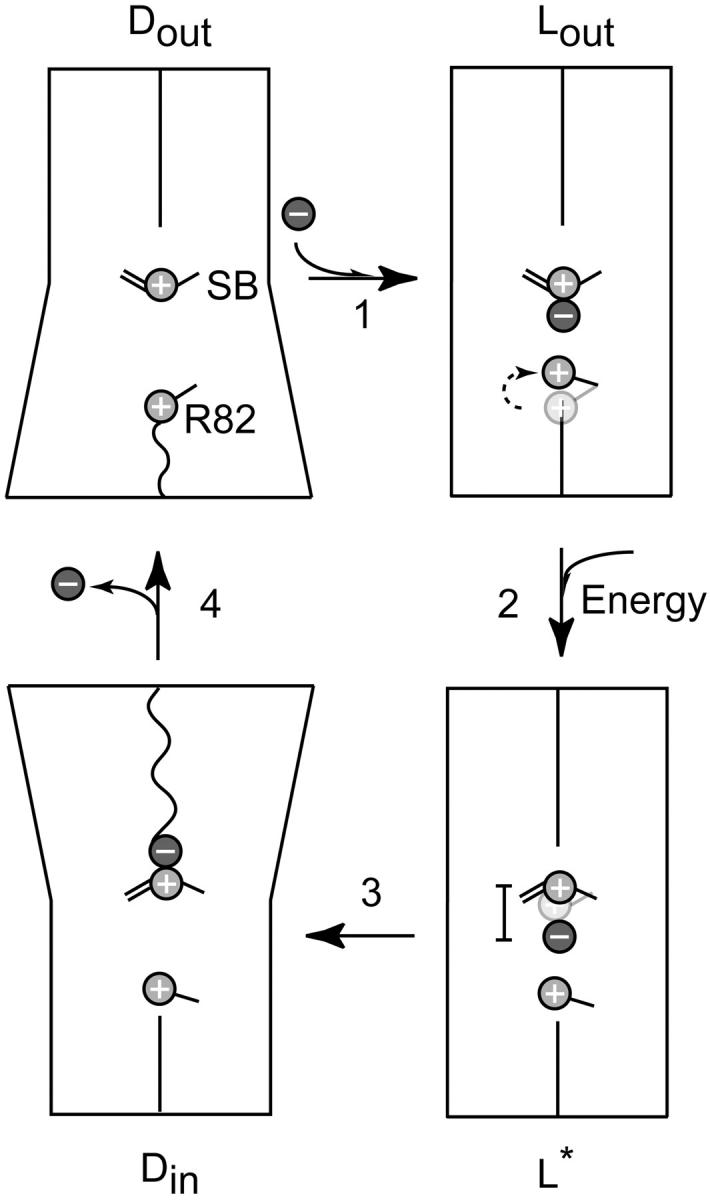
Model of the key functional steps in the ion-transport cycle of bR(D85S). The cartoon schematically shows, via sloping lines, an outward tilt for helices on either the extracellular side or the cytoplasmic side of the protein, producing the two types of dynamic states, (Dout and Din). The presumed increase in the permeability of the corresponding half of the protein is indicated by the transition from a straight to wiggly line crossing that half of the protein. In the first step, the extracellular half of the transport channel starts in a “dynamic” conformation, Dout. The side chain of R82 serves as a dynamic gate that, by fluctuating between inward facing and outward facing conformations, allows entry of a halide ion to the binding site. Substrate binding then induces a conformational transition to the “latched” state, Lout, in which R82 is held tightly in the upward facing position. The movement of R82 is indicated by a change in position of the schematic side chain and a curved dashed arrow. The initial position of the side chain is shown in faint gray. In the second step, photon energy is absorbed by the retinal chromophore and results in a transition to a high-energy state, L*, in which a small displacement of the protonated Schiff base relative to the bound halide raises the potential energy of the halide ion by a large amount. The displacement of the Schiff base is represented in a similar fashion to that of R82, whereas a bar indicates that this displacement increases the distance between the halide ion and the Schiff base. The third step envisions a repacking of helices on the cytoplasmic side of the protein that yields a dynamic state, Din, which allows the anion to exit the protein. Finally, in the fourth step, the protein relaxes back to its ground state, Dout.
