FIGURE 8.
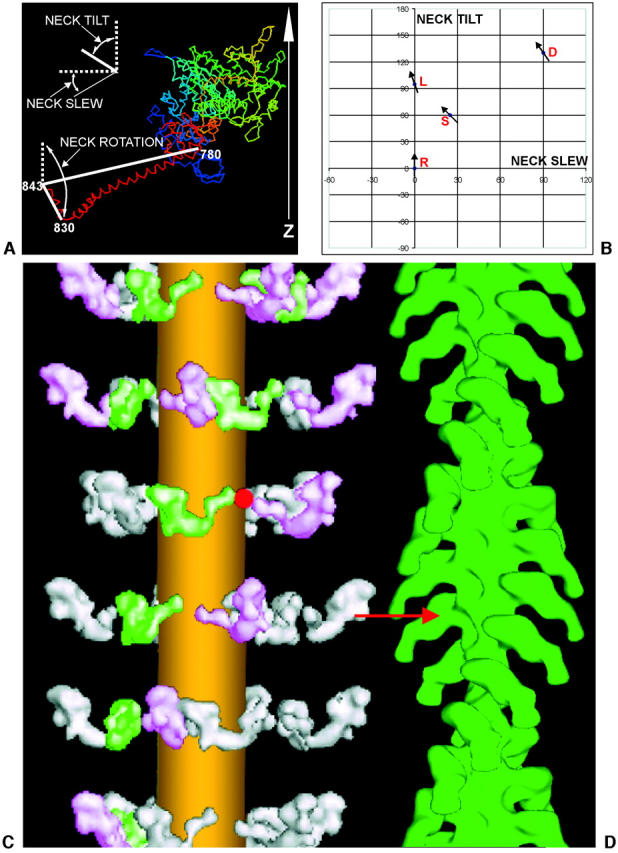
(a) Defining the different motions of the lever arm and hook axis denoted by neck tilt, neck slew, and neck rotation in different myosin head structures, assuming that the catalytic domain is oriented as it would be if attached to actin in the rigor conformation, with the actin axis vertical and the Z-band end at the bottom. The neck long axis is taken as the line joining residues 780 and 843, and the hook axis is taken as the line joining residues 830 to 843. (b) A plot showing the neck tilt and neck slew angles required by the lever arm in order to convert between the four structures when the catalytic domain is superimposed on a vertical actin filament in the rigor attachment position (see Fig. 7, a and b). The arrows on different points show the relative neck rotations of the hook region of the myosin head in the different structures. The vertical reference axis is the actin filament axis with M-band up, Z-band down. R refers to the Rayment rigor structure (Rayment et al., 1993a,b), D refers to the Dominguez ‘prepowerstroke’ structure (Dominguez et al., 1998), L is Lethocerus (this work), and S is the scallop myosin Mg•ADP•VO4 structure (Houdusse et al., 2000). (c and d) The relaxed to rigor transition involves a swing of the myosin neck domain. Our best, relaxed myosin thick filament model is shown on the left (c), and an actin filament reconstruction labeled with S1 in the rigor state is shown on the right (d) (Harford and Squire, unpublished data from S1-labeled fish muscle). For an actin target monomer ideally opposite a relaxed myosin head, the relaxed to actin-bound (prepowerstroke) transition requires only a radial movement of ∼2 nm and a small rotation of the catalytic domain to achieve rigor-like docking of the catalytic domain on the actin site (red arrow). After docking, only a purely axial swing (no slew), with a small pivot rotation (of +20°), of the myosin head neck domain (lever-arm) is needed to complete a powerstroke and to reach the final rigor conformation (as in Fig. 7, c and d). The lateral separation of the myosin and actin filaments has been exaggerated in this diagram.
