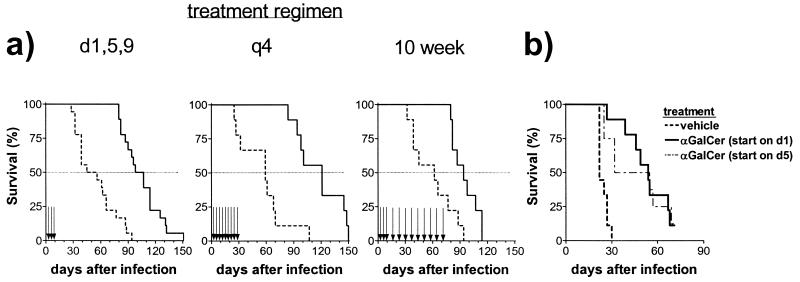
FIG. 3.
Chronic administration of αGalCer provided no additional survival benefit. BALB/c mice were inoculated intravenously and then administered vehicle or αGalCer. (A) In the standard protocol, vehicle or αGalCer was administered on days 1, 5, and 9 (P < 0.0001); in the q4 protocol, vehicle or αGalCer was administered every 4 days for a total of nine doses (P = 0.0004); in the 10-week protocol, vehicle or αGalCer was administered on days 1, 5, and 9 and then once a week for 10 weeks (P = 0.0031). (B) BALB/c mice were inoculated intravenously with M. tuberculosis and then divided into groups of nine mice. Two groups were treated by the standard protocol and received αGalCer or vehicle on days 1, 5, and 9 following infection. Another group was treated with αGalCer on days 5, 9, and 13, and the fourth group was treated on days 8, 12, and 16. The groups that received αGalCer starting on day 1 (P < 0.0001) or day 5 (P = 0.0013) had a prolonged survival compared to vehicle-treated controls.