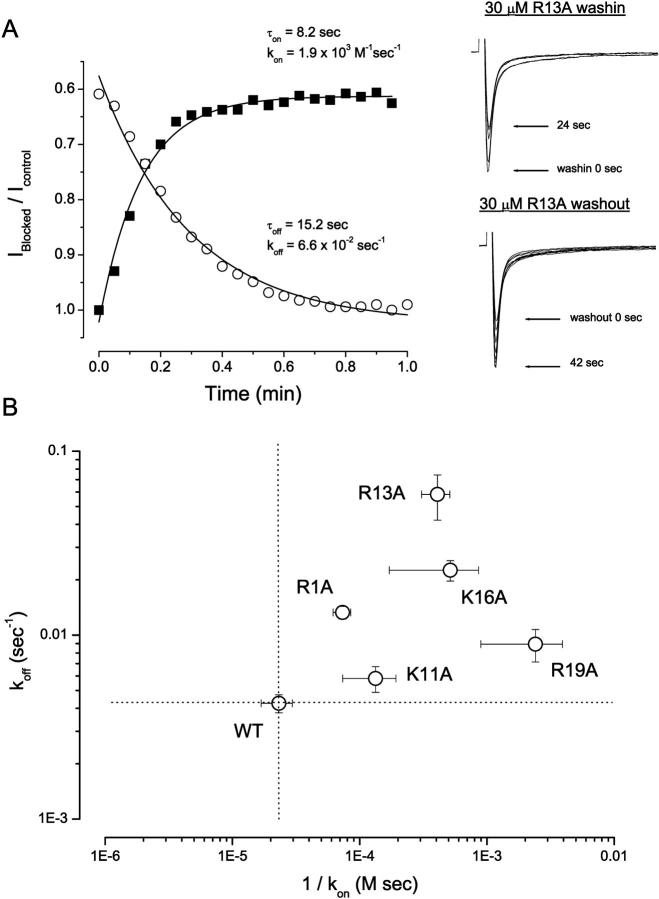
FIGURE 2.
Kinetic analysis of block by mutant μ-CTX derivatives. (A) Left Panel: Time courses of onset and offset of R13A (30 μM) block of WT μ1 channels during toxin wash in (solid squares) and wash out (open circles). Normalized peak sodium currents elicited by depolarization to −10 mV from a holding potential of −100 mV were plotted versus time. Data were fitted with a mono-exponential function to estimate τon and τoff for toxin binding and unbinding respectively. The equations used for deriving kon and koff from τon and τoff have been previously described (Li et al., 2000). Right Panel: Representative records of μ1 currents elicited during wash in (top) and wash out (bottom) of R13A (30 μM). Sweeps (15 msec duration) shown were separated by 6-s intervals for clarity. (B) Logarithmic plot of the dissociation rate constants (koff) versus the reciprocal of the association rate constants (kon). The horizontal and vertical dotted lines respectively represent the levels of 1/kon and koff for the WT channels. All mutant toxins studied (except K11A) had substantial effects on both kon and koff (see text for details). The largest effects on kon and koff were found with R19A and R13A, respectively.