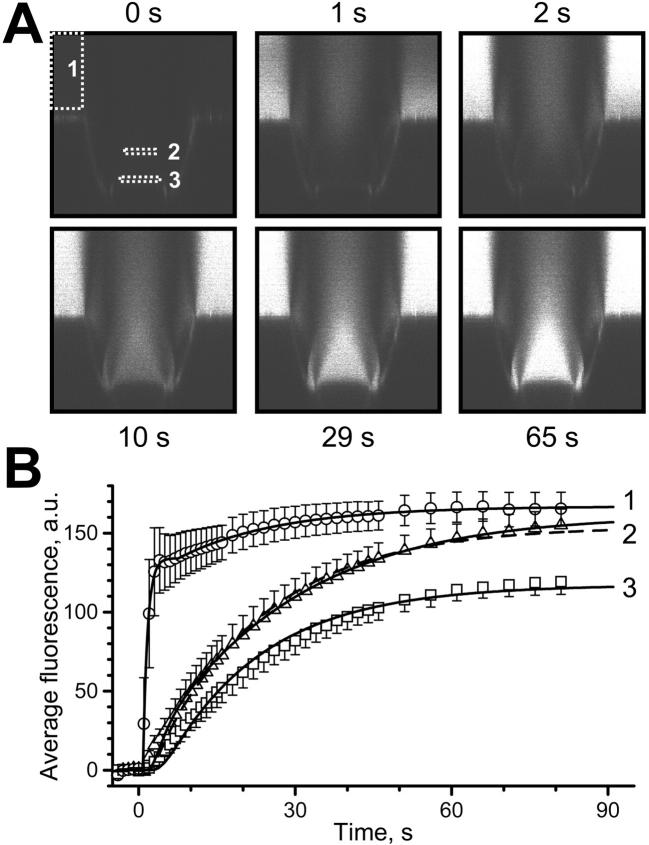
FIGURE 3.
Solution change kinetics and diffusion: experiment and theory. At t = 0, a solution containing 4 μM GFP and 100 mM sucrose was injected into a buffer-filled OSTR chamber. A series of vertical confocal 125 × 125 μm scans was acquired, employing the time lapse program of the microscope. (A) Examples of the image series at indicated times. White rectangles show regions where fluorescence was quantified. (B) Quantification and fit. Experimental data (symbols, average of six measurements, bars indicate SE) were derived from image series shown in A. Curve 1 represents best fit to solution change kinetics at the surface of the TC (box 1) yielding (in fluorescence units) C0 = 0, C1 = 134, C2 = 32.9, τ1 = 0.870 s, τ2 = 19.4 s, dt1 = 0.788 s, dt2 = 7.59 s in Eq. 18. Curve 2 represents best fit to experimental concentration change in area 2 (x = 30 μm) at different conditions: solid line: unconstrained fit was obtained with τD = 53.4 s, x/L = 0.48, and ratio of amplitude to the amplitude of curve 1 equals 0.97; dashed line: fit with fixed x/L = 0.6 yielded τD = 39.6 s and amplitude ratio 0.92. Curve 3 shows fit to experimental concentration change at the TC bottom (x = 50 μm) giving τD = 34.1 s and amplitude ratio 0.70.