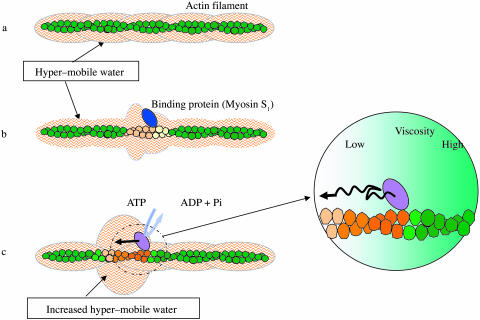
FIGURE 7.
A diagrammatic representation of the idea underlying the hypothesis of unidirectional sliding of myosin along F-actin. (a) The surface of F-actin is covered by a layer of hyper-mobile water (for simplicity's sake, the ordinary restrained water is not shown). (b) Upon interaction with a specific binding protein, actin changes its conformation, which is in turn propagated directionally to adjacent monomers to generate solvent space of axially skewed viscosity. (c) During an ATP hydrolyzing cycle, myosin undergoes conformational changes in coupling with alternating strong and weak affinities for actin. The conformational changes are transmitted to actin and propagated as in b. Thus, the asymmetric space for diffusion is dynamically generated, where myosin moves toward the direction of lower viscosity.