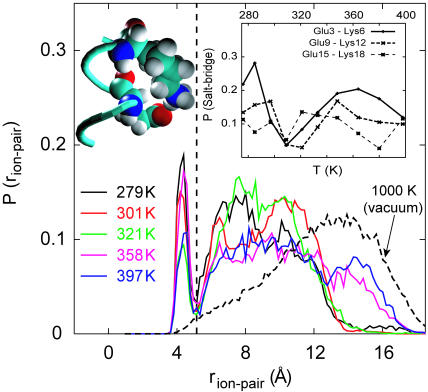
FIGURE 2.
Distribution of distances between the carboxylate carbon atom on Glu and amine nitrogen atom on Lys, averaged over the three “i, i + 3” (E,K) pairs in the EK peptide. Plots are shown for T = 279 K, 301 K, 321 K, 358 K, and 397 K obtained from REMD simulations and T = 1000 K obtained from an MD simulation of the uncharged peptide in vacuum. Snapshot of a typical Glu9-Lys12 contact/salt-bridge configuration at T = 279 K is also shown. Inset shows the probability of salt-bridge formation as a function of temperature obtained from REMD simulations of the EK peptide plotted separately for each of the three “i, i + 3” (E,K) pairs.