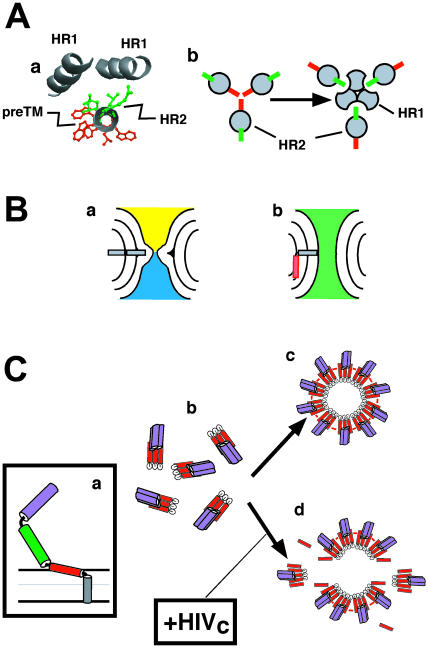
FIGURE 9.
Putative roles of interfacial hydrophobicity at gp41 preTM region. (A) Assembly of N-terminal amphipathic preTM region would hinder HR1-HR2 interactions. (a) Helical model for HIV-1 gp160 652QQEKNEQELLELDKWASLWNWFNIT676 sequence (underlined residues are part of the gp41 HR2 domain). Green side chains denote the residues participating in hydrophobic packing with HR1 helices in the gp41 six-helix bundle crystal structure. Red side chains belong to hydrophobic-at-interface residues of the N-terminal subdomain in the preTM. (b) Relative orientation of residues participating in hydrophobic packing with HR1 helices (green) and hydrophobic-at-interface residues (red) in prefusion (left) and postfusion structures (right). (B) Differential increase of monolayer surface by interfacial preTM may help fusion pore opening. A fractured TMC (a) might be brought to opened fusion pore (b) after hydrophobic-at-interface preTM (red) insertion into cis-monolayers. (C) Formation of fusion-competent gp41 complexes at the membrane surface. Fusion activation would lead to preTM insertion (a). Gp41 trimers (b, top view) would assemble into fusion-competent arrangements stabilized through preTM interactions (c, top view). Externally added HIVc would interfere with the assembly of functional complexes in membranes (d, top view). PreTM, HR1, and HR2 are designated in red, purple, and green, respectively.