Abstract
In mixed alcohol-water solvents, bovine β-lactoglobulin undergoes a cooperative transition from β-sheet to a high α-helix content conformer. We report here the characterization of β-lactoglobulin by compressibility and spectroscopy measurements during this transconformation. Both the volume and compressibility increase as a function of alcohol concentration, up to maximal values which depend on the chemical nature of the three alcohols used: hexafluoroisopropanol, trifluoroethanol, and isopropanol. The order of effectiveness of alcohols in inducing the compressibility transition is identical to that previously reported for circular dichroism and thus independent of the observation technique. The highly cooperative sigmoidal curves found by compressibility determination match closely those obtained by circular dichroism at 222 nm, indicating a correlation between the two phenomena measured by the two different techniques. The presence of an equilibrium intermediate form was shown by the interaction of β-lactoglobulin with 8-anilino-1-naphthalene sulfonic acid, a probe widely used to detect molten-globule states of proteins. It was correlated with the plateau region of the volume curves and with the inflexion points of the sigmoidal compressibility curves. Ultrasound characterization of proteins can be carried out in optically transparent or nontransparent media.
INTRODUCTION
Although correct protein folding requires an unordered polypeptide chain to assume the structure resulting from evolution, there is now ample evidence that biologically relevant, non-native conformational states are present in the cell (Kuwajima and Arai, 2000). Such states may include fully unfolded conformations and partially unfolded conformations stable at equilibrium, as during translocation across biological membranes (Bychkova et al., 1988). Alternatively folded protein conformations are of interest because by accumulating and aggregating, they contribute to the pathogenesis of debilitating diseases such as Alzheimer's and Parkinson's associated with ageing, or to prion disease, a fatal neurodegenerative disorder. In the prion protein, the abnormal isoform of the protein is characterized by a shift from an α-helical to a β-pleated sheet structure (Raso and King, 2000).
Because proteins are able to adjust their conformation to alterations of their microenvironment, cosolvents are commonly used to induce conformational shifts from native to non-native states. For example, a number of investigators have studied the conformational transition of β-lactoglobulin (BLG) in the presence of alcohols, the most frequently used being hexafluoroisopropanol (HFIP), trifluoroethanol (TFE), and isopropanol (IPA). These drive the β-sheet-to-α-helix cooperative transition and serve as a model. The mechanism of this transition is considered to be a key issue in understanding the folding of a number of proteins (Dufour et al., 1990, 1993; Hirota et al., 1997; Kuwata et al., 1998; Gast et al., 2001), the accumulation of aggregated proteins, and the development of novel therapeutic strategies to prevent or cure long-term degenerative changes.
BLG, a major component of bovine milk, is a 36,800 molecular mass dimer in aqueous solution, each monomer containing eight antiparallel β-strands (51%) and one α-helix (7%). The molecule, however, displays an intrinsic preference for an α-helical structure. Secondary structure analysis from amino acid sequence predicts 48% of α-helix and 13% of β-sheet (Shiraki et al., 1995), suggesting a high degree of structural flexibility. At pH 2.0, the molecule splits into monomers, keeping a fully native structure as shown by nuclear magnetic resonance (NMR) characterization (Kuwata et al., 1998, 1999). The central cavity of the protein (calyx), closed by a loop, binds a variety of hydrophobic molecules, although the precise function of the protein remains unknown (Brownlow et al., 1997).
During the transition from the native β-sheet to the non-native α-forms of BLG in presence of alcohols, the surface of the protein in contact with the solvent is altered, as a structural and thermodynamic response to the new solvent conditions. With the increased alcohol concentration, a number of previously water-exposed residues will become buried within the protein and inaccessible to the solvent, while a number of buried residues will become exposed to the solvent, experiencing a more and more hydrophobic microenvironment. Thus the solvent-induced rearrangement leads to a complex redistribution of water and alcohol molecules between the protein hydration shell and bulk phase, especially in hydrophobic domains, which stabilize the native structure. During the transition, a number of investigators have described the presence of a molten-globule type (Kuwajima, 1989, Christensen and Pain, 1991) equilibrium intermediate, stabilized by organic solvents (Hamada and Goto, 1997, Mendieta et al., 1999), at concentrations depending on the chemical nature of the alcohol (Hirota et al., 1997).
To shed light on this mechanism, we have measured the change in compressibility accompanying the β-to-α transition in BLG in the presence of the three chemically distinct alcohols HFIP, TFE, and IPA, which were used in earlier conformational studies (Gast et al. 2001, and references within). Since little is known about compressibility variation during such extensive conformational transitions, our aim was to correlate the compressibility measurements with conformational studies. Comparison of circular dichroism and compressibility data obtained in the same solvent indicates a close correlation between the highly cooperative increase of helicity and that of compressibility.
Moreover, the presence of an intermediate form is deduced from the binding to BLG of 8-anilino-1-naphthalene sulfonic acid (ANS), a fluorescent probe which is almost standard for diagnosing conformationally mobile partially folded compact states, referred to as molten globules (Semisotnov et al., 1991). We have correlated the intermediate form with the volume and compressibility curves by simple data analysis. In addition, we observed a variation of energy transfer from one of the tryptophan residues to the neighboring ANS binding site in the calyx, a transfer which is affected by the conformational transition.
MATERIALS AND METHODS
Materials
Bovine milk β-lactoglobulin (L 3908) purchased from Sigma (St. Louis, MO) was used without further purification. Isopropanol (IPA), 99.9% pure, was Lichrosolv grade from Merck (Darmstadt, Germany). Trifluoroethanol (TFE) and hexafluoroisopropanol (HFIP), both 99% pure, were obtained from Sigma, as well as 8-anilino-1-naphthalene sulfonic acid (ANS). All the other chemicals were analytical grade. Water used in this study was of Milli-Q purity.
Sample preparation
BLG was dried under vacuum and the samples prepared by weighing the dry lyophilized proteins on a Sartorius Model 1712 balance (Sartorius, Gottingen, Germany) with a precision of ± 0.03 mg, in volumetric flasks (class A ± 0.04 ml). The protein concentration (cp) of the samples was ≈3 mg ml−1. The solvents used to make up the volume at 20°C were mixtures of the alcohol with the appropriate amount of water, brought to pH 2.0, with HCl.
Volumetric measurements
The densities of solvents ρ1 and protein solutions ρ were determined at 25.00 ± 0.01°C, using a vibrating tube Anton Paar DMA 58 digital density meter (Anton Paar, Graz, Austria). The precision obtained in density measurements is >10−5. Each set of determinations was carried out at least 5×, averaged, and the value used to calculate the protein apparent specific volume ϕv (see Eq. 4). Because of the low solubility of BLG at the highest alcohol concentration, the measurements were stopped at alcohol concentrations where the maximum amount of α-helix was reached (Hirota et al., 1997), after checking that the solution was devoid of any precipitate.
Ultrasound velocity measurements
We have selected a method based on time of flight determination which offers, in addition to its simplicity, a high precision permitted by recent instrumental and computational advances (Amararene et al., 1997).
Principle
In ultrasound velocity measurements using the time of flight, a short electrical pulse is applied to a piezo-electrical transducer, converting the electrical wave into an acoustical one, which propagates through the medium under study. A second transducer at the end of the cell converts the received wave back into an electrical signal, which is compared to the excitation signal. The time interval between the two signals allows the determination of the ultrasound velocity, with knowledge of the precise distance between the two transducers.
Measurement system
One of the principal limitations of the custom-built system resides in possible temperature drifts. This difficulty is circumvented by the use of a set of tandem cells of identical acoustic path, enclosed in a single metal thermostated block (Sarvazyan, 1982), and experiments are carried out in a temperature-controlled room. The setup allows the sequential determination of the ultrasonic velocity difference between a reference and a measuring cell, which at identical temperature achieves a considerable improvement in sensitivity. First, the two cell acoustic paths are filled with the reference liquid, and velocity measurements are carried out. In a second step, the measuring cell is drained, rinsed, dried, and refilled with the liquid under investigation. The shift in the velocity occurring between the two cells over a period of 12 h is of the order of 2 cm s−1. The final precision in ultrasound velocity determination is >10−5 (Le Huérou et al., 2003).
Volume and compressibility calculation
Taking into account the relative densities of the protein solution (ρ), of the solvent (ρ1), that of the proteins (ρp), and their respective volume fractions (φ1), (φp), one can write from mass conservation law
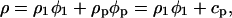 |
(1) |
where cp is the protein concentration. The values of ρ, ρ1, and cp are experimentally measured.
Since
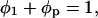 |
(2) |
Eq. 1 becomes
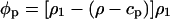 |
(3) |
and the protein apparent volume is
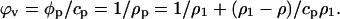 |
(4) |
The experimental measurement of the protein solution density (ρ) and that of sound velocity (u) allow the determination of the solution adiabatic compressibility using Laplace's equation.
Thus, the adiabatic compressibility of the reference solvent β1 can be written as
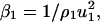 |
(5) |
where u1 is the velocity in the reference solvent and ρ1 its density.
In this work we make use of the effective medium theory (Eq. 6), since the relevant acoustic wavelength is always orders-of-magnitude larger than the protein size and the solvent density does not differ much from the protein density (Pinfield et al., 1995). We take the solvent as phase one, whereas the second phase consists of protein solution. We describe each of the constituent components, as a function of the relative volumes of the constituent phases, in terms of several parameters. Thus, we can relate the protein solution compressibility β to both solvent (β1) and protein (βp) compressibilities as
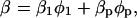 |
(6) |
where β, β1, and φp are calculated from ρ, ρ1, cp, u, and u1.
The protein compressibility can then be written as
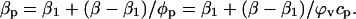 |
(7) |
The data were plotted versus the alcohol concentration and fitted to sigmoids.
Circular dichroism
Circular dichroism (CD) spectra were recorded using an Aviv Model 62A spectropolarimeter (Aviv Associates, Lakewood, NJ). The temperature of the sample was controlled at 25°C ± 0.1°C. In the far ultraviolet (UV), a 0.2-cm pathlength cuvette was employed. The results are expressed as mean residue ellipticities, defined as [θ] = 100 [θ]obs/lc, where [θ]obs is the observed ellipticity in degrees, c is the concentration in residue mole liter−1, and l is the length of the cell light path in centimeters.
Fluorescence
BLG intrinsic fluorescence and ANS fluorescence were recorded on a Spex spectrofluorimeter (Instruments SA, Edison, NJ) at 22°C, using excitation and emission bandpass of 2 nm. The excitation wavelengths for tryptophan and ANS fluorescence were 295 and 370 nm, respectively. Emission was recorded in the 310–570-nm wavelength range at a speed of 1 nm/s. In ANS binding studies, the dye concentration was 25 μM in a ratio of 1:1 to the protein at pH 2.0. The alcohol concentration in the samples was varied in a 0–40% range depending on the chemical nature of the alcohol.
RESULTS
Volume
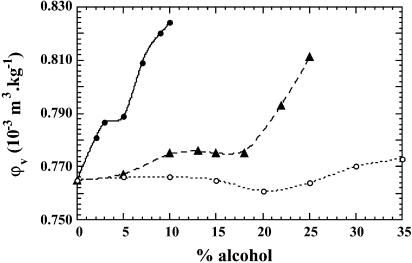
The dependence of partial specific volume of BLG on alcohol concentration is shown in Fig. 1. In all three alcohols the maximum variation is modest, within 10%. The curves determined in the two fluorinated alcohols present a somewhat similar overall profile, consisting first of a volume increase, then of a plateau region, and finally a steep increase. In HFIP, the protein volume increases from 0.765 10−3 m3 kg−1 in water at pH 2.0, to 0.788 10−3 m3 kg−1 at 3% HFIP, then we observe a short plateau between 3 and 5% (midpoint at 4%), followed by a much steeper increase, reaching a value of 0.824 10−3 m3 kg−1 at 10% HFIP. In TFE, the volume of BLG increases slowly from 0.765 to 0.772 10−3 m3 kg−1 at low cosolvent concentration (between 5 and 10%), then the curve displays a plateau between 10 and 18% of alcohol (midpoint at 14%), followed by an ascending part starting at 20% and reaching a value of 0.812 10−3 m3 kg−1 at 25%. In contrast, when IPA is the co-solvent, the volume curve of BLG is almost flat, at a value of 0.765 10−3 m3 kg−1 in the range from 0 to 15%; declines to a minimum at 20%; and then shows a very slowly ascending part at alcohol concentration from 20% to 35%, finally reaching a value of 0.772 10−3 m3 kg−1 at 35%. Thus Δv, the maximum difference in volume values, is cosolvent-dependent: 0.059 10−3 m3 kg−1 (1086 ml/mol) in HFIP, 0.047 10−3 m3 kg−1 (865 ml/mol) in TFE, and only 0.007 10−3 m3 kg−1 (129 ml/mol) in IPA. Remark that the numbers in the fluoroalcohols are extremely large for protein volumetric changes.
FIGURE 1.
Plot of φv, the apparent partial specific volume of BLG, as a function of alcohol concentration (v/v %), in water at pH 2.0. In this and all subsequent figures, • represents experiments carried out in HFIP, ▴ in TFE, and ○ in IPA. The error in volume measurements is ± 0.003 10−3 m3 kg−1.
Compressibility
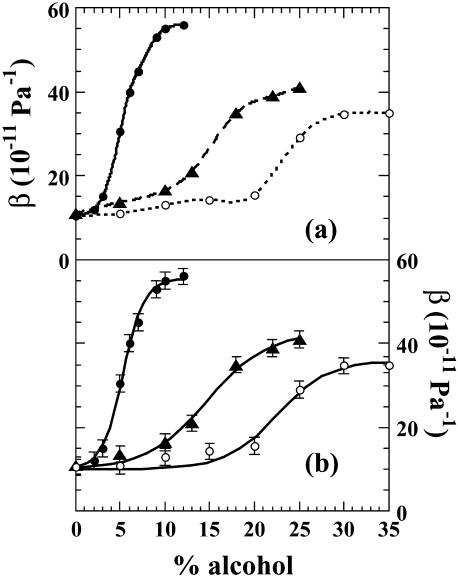
When BLG adiabatic compressibility, β, is plotted versus alcohol concentration expressed as volume percent (v/v), a smooth sigmoidal curve is obtained indicating a cooperative transition from low to high compressibility values (Fig. 2 b). It is interesting that the maximum compressibility value reached varies also with the chemical nature of the alcohol. Thus whereas in TFE and IPA mixtures the compressibility value levels off at a maximum between 35 to 40 × 10−11 Pa−1, it reaches a substantially higher value of 55 × 10−11 Pa−1 in HFIP solutions (Fig. 2 a). Furthermore, the alcohols differ in their effectiveness to induce the compressibility transition in the order: HFIP > TFE > IPA, the most effective being the fluorinated alcohols, exactly as previously reported by CD measurements of the transition (Hirota et al., 1997). We obtain for the maximum difference in compressibility a value of 45 × 10−11 Pa−1 in HFIP, 30 × 10−11 Pa−1 in TFE, and 23 × 10−11 Pa−1 in IPA in decreasing order. From the fitted sigmoids we obtain the inflexion points of the curves at 5, 15, and 21%, of HFIP, TFE, and IPA, respectively.
FIGURE 2.
(a) Plot of β, the adiabatic compressibility of BLG, as a function of alcohol concentration. (b) Compressibility data fitted to sigmoids. The error bar in compressibility measurements is ± 2 10−11 Pa−1.
Circular dichroism
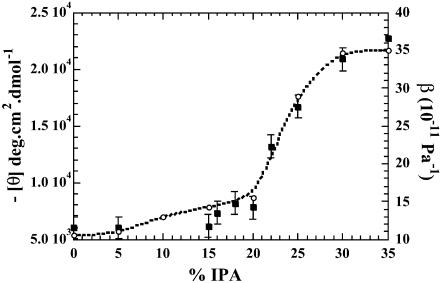
To investigate the β-sheet to α-helix transition of BLG, a large number of conformational studies have been previously carried out in mixtures of fluorinated alcohols (Shiraki et al., 1995, Hamada and Goto, 1997; Hirota et al., 1997; Kuwata et al., 1998; Hong et al., 1999; Mendieta et al., 1999; Gast et al., 2001). Here we measured the conformational change of the protein in IPA, the least effective of the three cosolvents in inducing the transition, as this alcohol's effects on the CD of BLG have been least-studied. Titration of the protein with IPA was monitored by ellipticity at 222 nm. The resulting curve shows the induction of helical conformation in BLG (Fig. 3). The compressibility of BLG in IPA has been plotted at the same time. It is interesting that the curves both display the same sigmoid profile and almost overlap, indicating a close parallelism between the increase of the α-helix amount and that of the protein compressibility. Two differences can, however, be observed between the curves. Firstly, the far-UV CD curve is almost flat, up to an alcohol concentration of 15%, whereas the compressibility values start to increase slowly at a concentration of 5%. Secondly, a careful inspection of the CD curve in IPA reveals a notch between concentrations of 15 and 18%, whereas the compressibility curve does not show this phenomenon.
FIGURE 3.
Plot of ellipticity [θ] at 222 nm, on the left (▪), and adiabatic compressibility β of BLG on the right (○), versus isopropanol concentration. Observe the notch on the CD curve between 15 and 20% of IPA.
Fluorescence
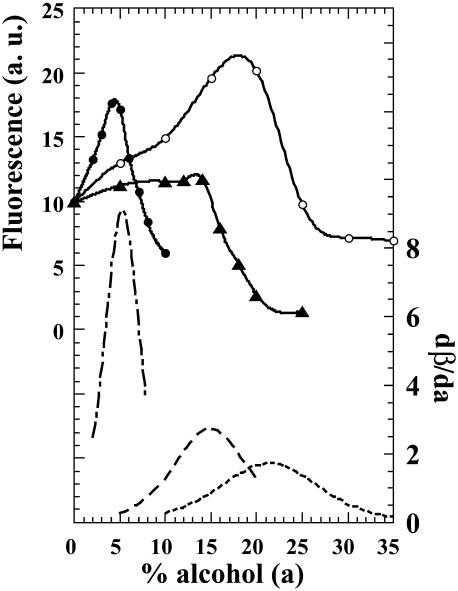
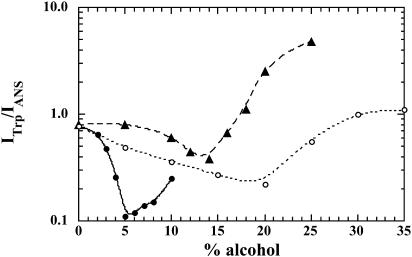
Since literature reports the presence of an equilibrium molten-globulelike structure at low alcohol concentration, we have explored the binding to BLG of the fluorescent probe ANS, a standard for diagnosing molten globules (Semisotnov et al., 1991; Uversky et al., 1997), as a function of alcohol concentration (Fig. 4). After binding, ANS fluorescence emission intensity increases up to a maximum reached near 4% in HFIP, near 14% in TFE, and between 18 and 20% in IPA, and then drops abruptly. The derivatives of the compressibility curves were also plotted on Fig. 4. The maxima in the derivative curves correspond, of course, to the inflexion points of the sigmoids. The correlation between these and the fluorescence maxima of the ANS complexes is excellent. In addition, we observed a nonradiative energy transfer between one of the two tryptophans, excited at 295 nm and ANS bound within the calyx. When the ratio of tryptophan fluorescence intensity over that of ANS is plotted versus alcohol concentration, we obtain three curves displaying similar profiles (Fig. 5). First there is a decrease in the ratio down to a minimum, and then a progressive increase. The minimum is located at different alcohol concentrations depending, as already observed in this report, on its chemical nature (Fig. 5). We found the minima at concentrations of 5% of HFIP, ∼14% of TFE, and ∼20% of IPA.
FIGURE 4.
Plot of ANS fluorescence intensity in arbitrary units, versus alcohol concentration (a). The protein and dye concentration were 25 μM. The excitation wavelength is 370 nm. The dotted lines correspond to the derivate dβ/da, and the maxima to the inflexion points of compressibility curves: HFIP (–·–·–), TFE(- - - -), and IPA (·····).
FIGURE 5.
Plot of energy transfer efficiency between tryptophan and ANS. The ratio of tryptophan to ANS fluorescence intensity, ITrp/IANS, is plotted versus alcohol concentration. The excitation wavelength is 295 nm.
DISCUSSION
Although recent developments of acoustic techniques allow a much higher precision in measurements by difference ultrasound velocimetry, there is a general agreement that adiabatic compressibility of globular proteins cannot be interpreted by the correlation with a single variable. As underlined by Dadarlat and Post (2001) in an article on molecular dynamic simulations of protein compressibility, “the compressibility is a complex function that may result from a combination of factors, such as protein packing density, hydrophobicity, polarity, specific solvent accessible area, number of disulfide bonds, and the types of secondary structure elements.” The fact that we are dealing in this work with a major change of protein structure induced by a series of cosolvents adds to the complexity of these parameters, and of data interpretation.
The precise mechanism by which alcohols induce conformational transitions in proteins and peptides is still a matter of conjecture and discussion. According to literature, alcohols may exert their effects by at least two mechanisms: direct binding or an indirect mechanism, by changing the solvent shell around the polypeptide chain (Kentsis and Sosnick, 1998). A superposition of these effects can thus be expected (Gast et al., 2001, and references within). In addition, the osmophobic effect described by Bolen and co-workers as an unfavorable interaction between the alcohol and the peptide backbone (Bolen and Baskakov, 2001) may be involved. Each of these hypotheses is plausible to some degree.
Actually, little is known about the precise structure of the α-helices induced by alcohols in BLG. From NMR experiments, Kuwata et al. (1998) reached the conclusion that the residues involved in the conformational changes are mainly located on the N-terminal half of the molecule. From small-angle x-ray scattering experiments, Shiraki et al. (1995) and Kamatari et al. (1999) concluded that the protein was no longer globular, but chainlike, with chain segments formed by noninteracting α-helices. Furthermore, the dimensions of other proteins studied in methanol appear to be closer to those in a highly unfolded state than globular. The protein could be represented by an open structure of helical segments without notable interactions between the segments. According to Gast et al. (2001), the structure of BLG in fluoroalcohols reveals a strongly solvated chain of different helical segments with several helical rods linked by flexible random coils, in agreement with the model proposed by Muroga (2000). We have characterized here the above structures by volume and compressibility measurements.
From our results there is a clear evidence that the maximum increase in volume depends on the chemical nature of the alcohols used and follows the order of alcohol effectiveness (HFIP > TFE > IPA) in inducing the transition (Fig. 1). It is important to note that the maximum alcohol concentration used in all the experiments corresponds to the plateau value of the ellipticity at 222 nm, i.e., 80% of α-helix (Hirota et al., 1997). Thus, despite displaying the same α-helix content, the final structures appear to differ in maximal volumes. For example, in the case of IPA where the cosolvent binding seems to be minimal (English et al., 1999), we might conclude either that the transition per se involves a relatively small volume change or that underlying positive and negative contributions almost cancel each other. A number of compensating effects may indeed affect the protein volume and contribute to the final result. The much larger values of Δv maximum found for HFIP and TFE compared to IPA indicate that the preferential interaction of the fluorinated alcohols with the protein seems to play a significant role in such high volume changes. This situation leads to a highly solvated protein described by Gast et al., (2001), where the excess concentration of fluorinated alcohols induces packing defects at the protein surface, significantly increasing the volume.
The primary focus of this investigation was to explore the extent to which the β-sheet-to-α-helix model transition is associated with changes in BLG adiabatic compressibility. Clearly, our results indicate a very close match between the dependence on alcohol concentrations of the ellipticity at 222 nm and that of the compressibility. This fact implies that the compressibility changes observed in this work do reflect the conformational transition between the BLG β-native and the open α-states (Fig. 3). An additional feature suggesting the close relationship between the protein cooperative conformational transition and the compressibility increase is the order of effectiveness of the alcohol species for inducing them (HFIP > TFE > IPA). We find it to be the same whatever the technique used: compressibility, volume or CD. Other factors may, however, contribute to an increase of protein compressibility: for example, the pH of the solution promoting aggregation. However, since the protein exists at pH 2.0 as a monomer, aggregation can be safely ruled out. In addition, the presence of the cosolvents increases the repulsive electrostatic interactions, characteristic of low pH, leading to increased interatomic distances and size of protein cavities and thus to the softening of the macromolecule. At present, we cannot quantify the respective contributions of the potential solvation by the alcohols at the protein surface and that of the conformational transition to the compressibility.
One notable difference between CD and compressibility experiments concerns the maxima reached. While it is known that the maximum α-helicity induced by alcohols (80%) is independent of the chemical nature of the cosolvent (Hirota et al., 1997), the maximum compressibility value obtained for BLG in HFIP is definitely higher than that in the two other alcohols, up to the concentration studied. This effect is consistent with the preferential binding coefficient of HFIP to BLG. It is probably the result of hydrophobic interactions and of the replacement of water by alcohol molecules leading to progressive protein unfolding (Gast et al. 2001). An additional difference between the CD and compressibility curves is the early increase of the compressibility values, which is absent in the far-UV CD curve (Fig. 3). This probably reflects structural changes observed in the near-UV CD, between 5 and 15% of IPA, where the aromatic fine peaks at 280–295 nm disappear (not shown). Another notable discrepancy between compressibility and CD curves is the presence of a notch in the [θ]222 versus IPA concentration curve (Fig. 3). This phenomenon has also been described by Konno et al. (2000) when titrating cytochrome c by HFIP. According to these authors, it indicates the presence of an intermediate state during the transition to a highly α-helical state. This notch in the titration curve of BLG with TFE is also present in the work of Mendieta et al. (1999), who used CD spectral deconvolution to pinpoint the conformation of the molten-globulelike intermediate.
Interaction with ANS identifies protein molten-globule states, using the term in a broad sense (Semisotnov et al., 1991). This is indeed the case here, since the intermediate state shares many features of the molten globule but differs nevertheless from the BLG native secondary structure (Shiraki et al., 1995). As shown in Fig. 4, in all the three cosolvents a strong enhancement of ANS fluorescence occurs upon interaction of the dye with the more loosely packed clusters of hydrophobic residues of the partially folded protein. According to Collini et al. (2000), the protein possesses two binding sites for ANS: one external site and another one more specific, at the bottom of the hydrophobic calyx, possibly close to the GH strand (Brownlow et al., 1997). The calyx site would certainly be affected by the conformational transition. At higher alcohol concentrations, the hydrophobic ANS binding pocket is destabilized. It appears to be solvated by the hydrophobic solvent, as indicated by the minima in the fluorescence energy transfer curves (Fig. 5). From the energy transfer observed, we can conclude that the ANS binding site within the calyx is within 20 Å of tryptophan 19 (Brownlow et al., 1997).
It is especially interesting that virtually all the measurements shown in this work display a relatively small transition at low alcohol concentrations followed by a much larger one at higher cosolvent levels. The early transition is seen to correspond to the loss of the protein's native tertiary structure without having large effects on the secondary structure, as seen with IPA in Fig. 3. This is indicative of classical molten-globule formation. With IPA this transition is complete at slightly <20% alcohol. The sharp increase in negative ellipticity at higher IPA levels, which corresponds to the large change in compressibility as well as to the change in volume, is accompanied by a loss of ANS fluorescence and thus of ANS binding. The same loss of ANS binding occurs with the other two alcohols as well, but at lower concentrations, as noted above. The highly α-helical form produced at elevated alcohol concentrations thus does not have the properties normally associated with molten globules, namely a compact form with a mobile but hydrophobic interior.
In addition to its role as a molten-globule indicator, ANS can also bind in the calyx of native BLG. This too, increases its fluorescence from a very small value in water to the value seen in Fig. 4 at 0% alcohol. The shoulder on the ANS fluorescence curve for IPA that occurs at ∼5% alcohol is consistent with additional changes in the binding, due perhaps to some softening of the native tertiary structure as it moves toward the molten-globule state. TFE behaves in a manner similar to IPA. It produces a small initial volume change (Fig. 1) as well as a small early rise in compressibility (Fig. 2), both of which are complete by 10% alcohol. Similarly, the ANS fluorescence rises to a plateau by 7–10% and then rises slightly to its maximum at ∼14% (Fig. 4). The effects of HFIP are slightly more complex than those of the other two alcohols because they are compressed into a narrow range of relatively low cosolvent concentrations. Thus the early transition is not seen in the compressibility data in Fig. 2, or the ANS fluorescence in Fig. 4. It is clearly present in the specific volume in Fig. 1, however, where it occurs as a step which is complete by 3% alcohol.
As a conclusion, it is interesting to remark that the midpoints of the volume plateaus, the minima of energy transfer, the maximum fluorescence of the ANS binding curves, and the inflexion points of the compressibility curve are in good agreement, occurring at similar alcohol concentrations. Finally, if we compare the inflexion points of the compressibility curves and the ANS fluorescence maxima in Fig. 4, we can conclude that the largest change in compressibility for a given increase in alcohol concentration occurs for the molten-globule-like state.
In this report, we have highlighted the parallelism between the conformational transition and compressibility changes. The immediate challenge of these findings is to extend such measurements to probe correct protein packing and folding. Nevertheless, to define the different parameters leading to the values reported here, we still lack pertinent models to predict the compressibility of the highly α-helical state induced in BLG by the cosolvents. We believe, however, that a detailed description of their effect on the protein compressibility is a step toward elucidating their molecular mechanism and dissecting the contributions that account for the protein stability and specificity in the folded state. An important practical point worth stressing is that compressibility experiments do not require optically transparent solutions. Ultrasound measurements of proteins can therefore be used to characterize their conformational shifts in a wide range of opaque media.
Acknowledgments
The authors are grateful to Dr. L. Bachner for reading the manuscript and to Dr. Roger Pain and Dr. P. C. Kahn for advice, helpful discussions, and critical comments. They thank Dr. S. Maiti for his assistance in the fluorescence experiments.
This work was in part supported by a grant from Evaluation-orientation de la Cooperation Scientifique (M00P03).
References
- Amararene, A., M. Gindre, J.-Y. Le Huérou, W. Urbach, and M. Waks. 1997. Water confined in reverse micelles: acoustic and densimetric studies. J. Phys. Chem. B. 101:10751–10756. [Google Scholar]
- Bolen, D. W., and V. I. Baskakov. 2001. The osmophobic effect: natural selection of a thermodynamic force in protein folding. J. Mol. Biol. 310:955–963. [DOI] [PubMed] [Google Scholar]
- Brownlow, S., J. H. M. Cabral, R. Cooper, D. R. Flower, S. J. Yewdall, I. Polikarpov, A. C. T. North, and L. Sawyer. 1997. Bovine β-lactoglobulin at 1.8 Å resolution. Still an enigmatic lipocalin. Structure. 5:481–495. [DOI] [PubMed] [Google Scholar]
- Bychkova, V. E., R. H. Pain, and O. B. Ptitsyn. 1988. The “molten globule” state is involved in the translocation of proteins across membranes? FEBS Lett. 238:231–235. [DOI] [PubMed] [Google Scholar]
- Christensen, H., and R. H. Pain. 1991. Molten globule intermediates and protein folding. Eur. Biophys. J. 19:221–229. [DOI] [PubMed] [Google Scholar]
- Collini, M., L. D'Alfonso, and G. Baldini. 2000. New insights on β-lactoglobulin binding sites by 1-anilinonaphthalene-8-sulfonate fluorescence decay. Protein Sci. 9:1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadarlat, V. M., and C. B. Post. 2001. Insights into protein compressibility from molecular dynamics simulations. J. Phys. Chem. B. 105:715–724. [Google Scholar]
- Dufour, E., and T. Haertlé. 1990. Alcohol induces changes of β-lactoglobulin-retinol binding stoichiometry. Prot. Eng. 4:185–190. [DOI] [PubMed] [Google Scholar]
- Dufour, E., C. Bertrand-Harb, and T. Haertlé. 1993. Reversible effects of medium dielectric constant on structural transformation of β-lactoglobulin and its retinol binding. Biopolymers. 33:589–598. [DOI] [PubMed] [Google Scholar]
- English, C., S. Done, L. S. D. Caves, C. R. Groom, and R. E. Hubbard. 1999. Locating interaction sites on proteins: the crystal structure of thermolysin soaked in 2% to 100% isopropanol. Prot. Struct. Funct. Genet. 37:628–640. [PubMed] [Google Scholar]
- Gast, K., A. Siemer, D. Zirwer, and G. Damaschun. 2001. Fluoroalcohol-induced structural changes of proteins: some aspects of cosolvent-protein interactions. Eur. Biophys. J. 30:273–283. [DOI] [PubMed] [Google Scholar]
- Hamada, D., and Y. Goto. 1997. The equilibrium intermediate of β-lactoglobulin with non-native α-helical structure. J. Mol. Biol. 269:479–487. [DOI] [PubMed] [Google Scholar]
- Hirota, N., K. Mizuno, and Y. Goto. 1997. Cooperative α-helix formation of β-lactoglobulin and melittin induced by hexafluoroisopropanol. Protein Sci. 6:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, D. P., M. Hoshino, R. Kuboi, and Y. Goto. 1999. Clustering of fluorine-substituted alcohols as a factor responsible for their marked effects on proteins and peptides. J. Am. Chem. Soc. 121:8427–8433. [Google Scholar]
- Kamatari, Y. O., S. Ohji, T. Konno, Y. Seki, K. Soda, M. Kataoka, and K. Akasaka. 1999. The compact and expanded denatured conformations of apomyoglobin in the methanol-water solvent. Protein Sci. 8:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentsis, A., and R. T. Sosnick. 1998. Trifluoroethanol promotes helix formation by destabilizing backbone exposure: desolvation rather than native hydrogen bonding defines the kinetic pathway of dimeric coiled-coil folding. Biochemistry. 37:14613–14622. [DOI] [PubMed] [Google Scholar]
- Konno, T., J. Iwashita, and K. Nagayama. 2000. Fluorinated alcohols, the third group of cosolvents that stabilize the molten-globule state relative to a highly denatured state of cytochrome c. Protein Sci. 9:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima, K. 1989. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins. 6:87–91. [DOI] [PubMed] [Google Scholar]
- Kuwajima, K., and M. Arai. 2000. The molten globule state: the physical picture and biological significance. In Mechanism of Protein Folding, 2nd Ed. R. H. Pain, editor. Oxford University Press, New York. 138–174.
- Kuwata, K., M. Hoshino, S. Era, C. A. Batt, and Y. Goto. 1998.α→β transition of β-lactoglobulin as evidenced by heteronuclear NMR. J. Mol. Biol. 283:731–739. [DOI] [PubMed] [Google Scholar]
- Kuwata, K., M. Hoshino, V. Forge, S. Era, C. A. Batt, and Y. Goto. 1999. Solution structure and dynamics of bovine β-lactoglobulin. Protein Sci. 8:2541–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Huérou, J.-Y., M. Gindre, W. Urbach, and M. Waks. 2003. Compressibility of nanoinclusions in complex fluids by ultrasound velocity measurements. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. In press. [DOI] [PubMed]
- Mendieta, J., H. Folqué, and R. Tauler. 1999. Two-phase induction of the nonnative α-helical form of β-lactoglobulin in the presence of trifluoroethanol. Biophys. J. 76:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroga, Y. 2000. Applicability of broken-rodlike chain to conformational analysis of polypeptide chain. Biopolymers. 54:58–63. [DOI] [PubMed] [Google Scholar]
- Pinfield, V. J., M. J. W. Povey, and E. Dickinson. 1995. The application of modified forms of the Urick equation to the interpretation of ultrasound velocity in scattering systems. Ultrasonics. 33:243–251. [Google Scholar]
- Raso, S. W., and J. King. 2000. Protein folding and human disease. In Mechanism of Protein Folding, 2nd Ed. R. H. Pain, editor. Oxford University Press, New York. 406–428.
- Sarvazyan, A. P. 1982. Development of methods of precise ultrasonic measurements in small volume of liquids. Ultrasonics. 20:151–154. [Google Scholar]
- Semisotnov, G. V., N. A. Rodionova, O. I. Razgulyaev, V. N. Uversky, A. F. Gripas, and R. I. Gilmanshin. 1991. Study of the molten globule intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers. 31:119–128. [DOI] [PubMed] [Google Scholar]
- Shiraki, K., K. Nishikawa, and Y. Goto. 1995. Trifluoroethanol-induced stabilization of the α-helical structure of β-lactoglobulin: implication for non-hierarchical protein folding. J. Mol. Biol. 245:180–194. [DOI] [PubMed] [Google Scholar]
- Uversky, V. N., N. V. Narizhneva, S. O. Kirschstein, S. Winter, and G. Lober. 1997. Conformational transitions provoked by organic solvents in β-lactoglobulin: can a molten globulelike intermediate be induced by the decrease in dielectric constant? Fold. Des. 2:163–172. [DOI] [PubMed] [Google Scholar]