Abstract
Diffusion of cardiac ryanodine receptors (RyR2) in lipid bilayers was characterized. RyR2 location was monitored by imaging fluo-3 fluorescence due to Ca2+ flux through RyR2 channels or fluorescence from RyR2 conjugated with Alexa 488 or containing green fluorescent protein. Single channel currents were recorded to ensure that functional channels were studied. RyR2 exhibited an apparent diffusion coefficient (DRyR) of 1.2 × 10−8 cm2 s−1 and a mean path length of 5.0 μm. Optimal use of optical methods for analysis of RyR2 channel function requires that RyR2 diffusion be limited. Therefore, we tested the effect of annexin 12, which interacts with anionic phospholipids in a Ca2+-dependent manner. Addition of annexin 12 (0.25–4.0 μM) to the trans side of bilayers containing an 80:20 ratio of phosphatidylethanolamine/phosphatidylserine decreased RyR2 diffusion in a concentration-dependent manner. Annexin 12 (2 μM) decreased the apparent DRyR 683-fold from 1.2–10−8 to 1.8 × 10−11 cm2 s−1 and the mean path length 10-fold from 5.0 to 0.5 μm without obvious changes in the conductance of the native bilayer or in activation of RyR2 channels by Ca2+ or suramin. Thus, annexin 12 may provide a useful tool for optimizing optical analysis of RyR2 channels in lipid bilayers.
INTRODUCTION
In the preceding report, we demonstrated the ability to image Ca2+ fluxes through single ryanodine receptor (RyR2) channels reconstituted in planar lipid bilayers (Peng et al., 2004). This capability permitted us to determine the position of a RyR2 channel in the bilayer. As described below, RyR2 exhibits significant lateral diffusion. Due to its random nature it is difficult to compensate for diffusional movement and this can complicate optical imaging of fluorescence related to channel activity. Consequently, a method for limiting lateral diffusion was needed to permit application of optical techniques to investigations of the function of RyR2 channels reconstituted in bilayers.
Prior work from the Hall and Haigler laboratories demonstrated that presence of annexins on one side of the bilayer decreased nonactin-induced currents, altered the conductance properties of alamethicin channels, and reduced the rate at which the hydrophobic anion, tetraphenylborate, crossed the bilayer (Sokolov et al., 2000). Annexins bind preferentially to anionic phospholipids (Gerke and Moss, 2002; Swairjo et al., 1994; Swairjo and Seaton, 1994) in a Ca2+-dependent manner and it was proposed that annexins, such as annexin 12, prevented small molecules from crossing the membrane by altering properties of the lipid bilayer, such as viscosity, lipid phase separation, or lipid curvature. Although it is not yet clear which, if any, of these effects is responsible for the actions of annexins observed by these workers, their findings did suggest the possibility that annexins might influence movement of integral membrane proteins within the plane of the bilayer. As described in this report, this has proven to be the case, as annexin 12 added to the trans side of the bilayer decreased lateral diffusion of RyR2 channels in a concentration-dependent manner. Importantly, preliminary studies do not reveal any alteration in RyR2 channel properties in the presence of annexin 12. Our results suggest that annexin 12, and perhaps other annexins, may prove useful for stabilizing the position of ion channels reconstituted in lipid bilayers and thereby enhance optical imaging of fluorescent signals related to ion channel function. These studies have been presented as an abstract (Peng et al., 2003).
MATERIALS AND METHODS
With the following exceptions, the materials and methods used for this work were identical to those described in the companion report.
Optical bilayer system
The optical bilayer system used in the studies is described in detail in Peng et al. (2004). A small trans chamber was attached via a glass capillary tube to the head stage of an Axopatch 1-B patch-clamp amplifier (Axon Instruments, Union City, CA). The head stage was, in turn, mounted on a 3-axis hydraulic manipulator (Narashige, East Meadow, NY), which was used to position the trans chamber within the cis chamber. A water immersion microscope objective (70×, 1.2 NA, Lomo Optics, Germantown, MD) was introduced in a horizontal orientation through the front wall of the cis chamber. Focusing was achieved by using a computer-driven stepper motor to adjust the position of the trans chamber relative to the objective. A small magnetic stir motor was mounted in the rear wall of the cis chamber, where it held a small stir bar used to mix the contents of the cis chamber.
Fluorescence was imaged using a Bio-Rad MRC 600 confocal system mounted horizontally on an optical bench. Light at 488 nm from an argon ion laser was coupled into the system through a single-mode optical fiber. After initial positioning, reflected light images of the bilayer obtained with the Bio-Rad system were used to fine focus on the plane of the bilayer. Scanning and image acquisition were controlled using Comos software (Bio-Rad). Image export and processing were accomplished using Confocal Assistant software (developed by Todd Clark Brelje) and Adobe PhotoShop (version 7.0). The x,y images presented in this report contain 768 × 512 pixels (0.12 μm/pixel) and required ∼1 s to acquire. The x-t linescans shown in Fig. 5 contain 512 lines and again required ∼1 s to acquire.
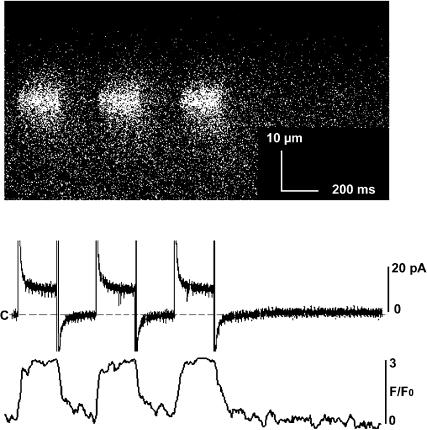
FIGURE 5.
Addition of annexin 12 to the trans side of the bilayer permits imaging of reproducible fluo-3 fluorescence signals. An x-t line scan image of fluo-3 fluorescence during repeated openings of a single RyR2 channel immobilized by addition of 2 μM annexin 12 to the trans side of the bilayer is shown in the top panel. RyR2 Ca2+ flux was elicited by three 200-ms voltage steps repeated at 200-ms intervals. Simultaneously recorded single RyR2 Ca2+ currents are presented in the middle panel and intensity-time traces derived from the fluorescent images are shown in the bottom panel. The image is 45 μm and 2 s in the horizontal and vertical dimensions, respectively. The reproducibility of the fluo-3 fluorescence during repeated linescans demonstrates the benefit of stabilizing the position of the RyR2 channel in the bilayer.
Preparation and use of annexin 12
Recombinant annexin 12 prepared as described previously (Patel et al., 2001) was stored as aliquots at −80°C at a concentration of 5 mg/ml. Before use, an aliquot was diluted with a solution containing 50 mM CaCl2, 10 mM Tris-Hepes (pH 7.4) to the desired final annexin 12 concentration and used as the trans chamber solution.
Preparation of fluorescent RyR2
RyR2 were labeled with Alexa 488 (Molecular Probes, Eugene, OR) using the following protocol. A quantity of 100 μl of a 1 M NaHCO3 solution (pH 8.0) was added to 1 ml of a sucrose gradient fraction containing RyR2 (see preceding report). A quantity of 50 μg of the succinimidyl ester of Alexa 488 carboxylic acid was dissolved in 15 μl of anhydrous dimethylsulfoxide and combined with the RyR2 gradient fraction. The resulting mixture was protected from light and incubated with agitation at 4°C for 2 h. Unreacted dye was removed by dialysis as described in the preceding report for reconstitution of RyR2 into liposomes with the exception that the first 1 L of dialysis buffer contained CHAPS (0.1% 3-[(cholamidopropyl)dimethylammonio]-propanesulfonate).
Recombinant mouse cardiac RyR2, RyR2D4365-GFP, in which the green fluorescent protein (GFP) had been inserted after Asp-4365, was prepared as described previously (Liu et al., 2002).
Characterization of the lateral diffusion of RyR2 in lipid bilayers
Fluo-3 fluorescent signals to single RyR2 channel currents were imaged in x,y scans every 3 s, the sequential full frame scan rate of the MRC 600 confocal system, in experiments conducted at room temperature. The location of RyR2 channels within the bilayer was established as the center of the fluorescence. This was determined by computing 2-dimensional median positions of pixels above a specified intensity level. Custom-designed software was developed using LabVIEW (National Instruments, Austin TX) to process time series of confocal images. The median position was selected to have equal numbers of suprathreshold pixels on either side of the location in both x and y directions. This scheme is relatively insensitive to individual stray pixels that might exceed the threshold value anywhere within an image. Median positions were determined from ∼5000 suprathreshold pixels within images containing a total of 400,000 K pixels.
Lateral diffusion coefficients (D) were calculated as described by Qian et al. (1991) using the relationship,
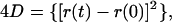 |
where r(t) and r(0) are the positions of a RyR2 channel at time t and time 0, respectively. The mean path length for diffusion of a RyR2 channel was calculated from the distances between each of the 20 positions determined during an individual experiment.
RESULTS
Lateral diffusion of RyR2 in bilayers
The ability to image fluorescence in response to Ca2+ flux through single RyR2 channels permitted us to track the position of this protein in the bilayer. As shown by the individual random walk diagrams in Figs. 1 and 2 and the cumulative data presented in Fig. 3, purified RyR2 reconstituted in lipid bilayers exhibit extensive lateral diffusion. An apparent lateral diffusion coefficient of 1.2 × 10−8 cm2·s−1 and a mean path length of 5.3 μm (n = 6) were observed for RyR2 channels in bilayers containing only phosphatidylethanolamine (PE). Since a goal of our work was to investigate the effect of annexin 12 on lateral diffusion of RyR2 channels and annexins bind to anionic phospholipids, such as phosphatidylserine (PS), we tested whether RyR2 diffusion was altered in bilayers containing both PE and PS. The presence of PS has been reported to decrease the mobile fraction of both lipid and recombinant syntaxin1A/SNAP25 in supported lipid bilayers in some studies (Wagner and Tamm, 2001). As shown by the example in the second panel of Fig. 1 and by the cumulative data in Fig. 3, addition of 20% PS to the bilayer did not significantly alter lateral diffusion of RyR2 channels. Lateral diffusion of RyR2 channels labeled with the fluorophore, Alexa 488, and of the recombinant RyR2-GFP chimera, RyR2D4365-GFP, was also assessed to confirm the results obtained by imaging fluo-3 fluorescence. A typical example for an Alexa 488-RyR2 channel is shown in Fig. 2. Similar results were obtained with all three approaches (data not shown).
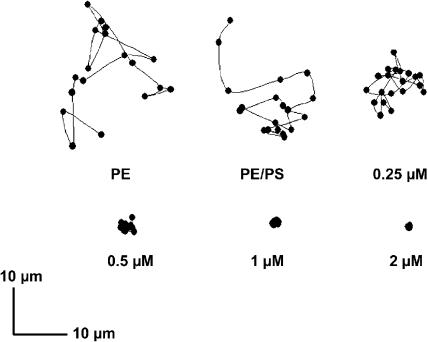
FIGURE 1.
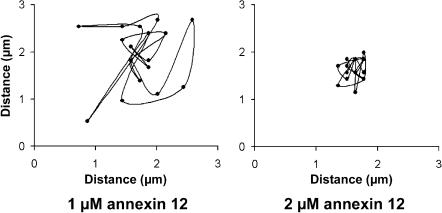
Lateral diffusion of a single RyR2 channel in a bilayer in the absence and presence of different concentrations of annexin 12. The position of the RyR2 channel was determined as the 2-dimensional median of the fluo-3 fluorescence response to Ca2+ flux through the channel. Twenty data points were recorded at 3-s intervals. In the left panel in the top row, the bilayer was composed of only PE. In the remaining panels bilayers contained 80:20 PE/PS. Annexin 12, at the concentrations indicated below the last four panels was added to the trans chamber before fusion of RyR2 and caused a concentration-dependent decrease in RyR2 diffusion. The dynamics of the diffusion of RyR2 channels and the immobilizing effects of annexin 12 can be appreciated from the movie provided as supplemental material to this report.
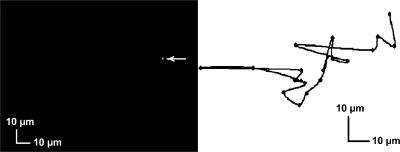
FIGURE 2.
Lateral diffusion of a single RyR2 channel labeled directly with Alexa-488. (Left panel) An x,y fluorescence image of a single RyR2 (indicated by the arrow). (Right panel) A random walk trace for this RyR2 channel (recorded at 3-s intervals in the absence of annexin 12). The results are similar to those obtained when fluo-3 fluorescence was used to track RyR2 position and for RyR2D4365-GFP.
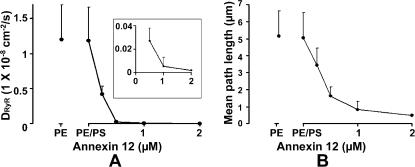
FIGURE 3.
Cumulative data showing effects of annexin 12 on the lateral diffusion of single RyR2 channels in bilayers. Data in these figures document further the ability of annexin 12 to reduce RyR2 diffusion in bilayers. The relationships between annexin-12 concentration and either the apparent lateral diffusion coefficient (DRyR) calculated for single RyR2 channels (left panel) or the mean distance moved by RyR2 during the 3-s imaging interval (right panel) are shown. Addition of PS to the bilayer in the absence of annexin-12 did not affect RyR2 diffusion. Results are means and SD of six determinations.
Annexin 12 diminishes diffusion of RyR2
We next assessed whether addition of annexin 12 to the trans chamber solution affected diffusion of RyR2 channels. As can be seen by comparing the random walk diagrams shown in the remaining panels in Fig. 1 and from the cumulative data presented in Fig. 3, the presence of annexin 12 on the trans side of the bilayer reduced lateral diffusion of RyR2 channels in a concentration-dependent manner. The extent of RyR2 channel movement in the presence of 1 and 2 μM annexin 12 is shown at a greater spatial resolution in Fig. 4. In the optical system used for these studies, pixels had a diameter of 0.12 μm and 20 data points were obtained at 3-s intervals. Thus, RyR2 were constrained to domains imaged by ∼±3.0 pixels over the course of 1 min in the presence of 2 μM annexin 12. However, since many photons are used to statistically determine RyR2 position, the spatial resolution is actually less than one pixel. The effects of 4 μM annexin 12 were assessed in two additional experiments and found to be similar to those produced by the 2-μM concentration. Thus, the effects of 2 μM annexin 12 may be maximal for the conditions used in the present studies. The usefulness of immobilizing RyR2 channels for optical imaging can be appreciated from the reproducibility of x-t scans made through the position of a RyR2 channel (Fig. 5). The dynamics of the diffusion of RyR2 channels in bilayers and the ability of annexin 12 to decrease this movement can be appreciated by viewing the movie provided as supplemental material to this report. When viewing the movie, differences in the intensity of fluo-3 fluorescence between experiments are evident. Measurements of RyR2 diffusion require only visible fluo-3 fluorescence; consequently, we did not attempt to evoke the same amplitude Ca2+ flux in every experiment.
FIGURE 4.
A pixel-by-pixel analysis of RyR2 diffusion in the presence of 1 and 2 μM annexin 12. In the optical system used for these studies, pixels had a diameter of 0.12 μm and 20 data points were obtained at 3-s intervals. Thus, RyR2 were constrained to domains imaged by ∼±3.0 pixels over the course of 1 min in the presence of 2 μM annexin 12.
Lack of effects by annexin 12 on bilayer conductance and RyR2 channel properties
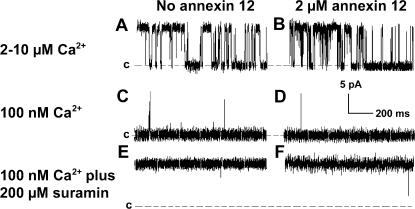
The ability of annexin 12 to limit RyR2 diffusion could make it a useful tool for optical analyses of the function of RyR2 channels reconstituted in lipid bilayers. However, such use requires that interactions between these proteins do not alter RyR2 channel properties. Although extensive studies are needed to address this question in a definitive way, preliminary observations suggest that annexin 12 does not exert marked effects on RyR2 gating and activation. RyR2 channels were activated to similar extents by 100 nM and 2–10 μM Ca2+ or by 100 nM Ca2+ plus 200 μM suramin in the cis chamber in both the absence and presence of 2 μM annexin 12 in the trans chamber (Fig. 6). Of equal importance, annexin 12 did not alter the conductance properties of the bilayer in the absence of RyR2 channels under the conditions used in the present studies.
FIGURE 6.
Annexin 12 does not alter activation of RyR2 channels by Ca2+ or suramin. The presence of 2 μM annexin 12 on the trans side of the bilayer does not alter activation of RyR2 channels by 2–10 μM (contaminating levels) of Ca2+ (current traces A and B), 100 nM Ca2+ (current traces C and D), or 200 μM suramin in the presence of 100 nM Ca2+ (current traces E and F). In each current trace the zero net current level is indicated by “c.” For current traces E and F this level is also indicated by a thin horizontal line below the trace.
DISCUSSION
In this report we describe the extent of lateral diffusion of single RyR2 channels reconstituted in lipid bilayers and the ability of annexin 12 to diminish this movement.
Lateral diffusion of RyR2 channels in bilayers
The ability of proteins to diffuse in both supported and unsupported bilayers is well established, and loss of channel activity in bilayer experiments is frequently attributed to movement of the channel from the bilayer to a thicker region in the surrounding torus. Consequently, when we first considered fluorescently imaging Ca2+ flux through RyR2 channels, we were concerned that a particle-tracking paradigm might be needed. Our hope was that the massive cytoplasmic domain of RyR2 would act as a sea anchor and retard its diffusion. Therefore, we were surprised and somewhat dismayed by the extent to which RyR2 diffuse in bilayers. A comparison of lateral diffusion coefficients reported in the literature for a variety of proteins reveals that RyR2 appear to diffuse as rapidly as smaller proteins. Such a comparison also shows the correlation between the molecular mass and lateral diffusion coefficient of proteins in lipid bilayers may not be particularly strong. However, our measurements cannot be viewed as quantitatively accurate for three reasons. First, due to the scan rates achievable with the Bio-Rad MRC 600 confocal system, we were only able to obtain x,y images of the bilayer and record the position of RyR2 channels every 3 s. A series of 20 data points were acquired for each record during a total interval of 1 min. As discussed by Qian et al. (1991) this measurement rate is too slow to permit accurate quantitation of diffusional movements. Consequently, the values we report for DRyR are only quantitatively accurate relative to each other and thus are referred to as apparent values.
Second, a question relevant to the relatively rapid rate of diffusion observed for RyR2 is whether the input of thermal energy associated with illumination of the bilayer required for fluorescence imaging causes an overestimation of the extent of RyR2 diffusion. This may be particularly problematic for laser scanning confocal systems, where a highly focused laser beam is used as the source of illumination. It is likely that the extent of heating may vary between experiments and this could be a partial cause of the somewhat large variability associated with our measurements. However, in this regard, it should be noted that by its nature diffusion is a highly variable event. The extent to which the intensity and type of illumination affect lateral diffusion can be assessed. This will require use of optical systems with different types of illumination and careful measurement of illumination intensities at the plane of the bilayer. In any case, the main point of the present report is that regardless of general quantitative accuracy, our measurements indicate clearly that even proteins as massive as the ∼2.3 × 106 Da RyR2 exhibit sufficient movement to complicate use of optical techniques for analysis of ion channel function in a bilayer system. It is necessary to restrict this movement to optimize both the spatial and temporal resolution of such analysis. Third, the torus region encircling the bilayer may act as a diffusion barrier in cases where RyR2 channels are present in peripheral regions of the bilayer. As considered by Qian et al. (1991), the presence of such barriers can influence quantitative aspects of diffusional movements.
In addition to diffusion, convectional flow within the bilayer could also contribute to the observed movement of RyR2 channels. We did not observe movements having a vectorial nature consistent with a convectional flow; however, the number of data points (20) collected during our experiments may not have been large enough to identify this type of motion. In addition, as noted above, the use of a laser light as the excitation light source is probably not the optimal approach to this question.
Annexin 12 decreases diffusion of RyR2 channels
As discussed in the Introduction, results from earlier studies suggested that annexin 12 altered properties of bilayers related to fluidity and stiffness (Sokolov et al., 2000). We reasoned that such properties could influence diffusion of integral membrane proteins, so we used the optical bilayer system to investigate whether the effects of annexin 12 would constrain movement of RyR2 channels under the experimental conditions used in our studies. This was found to be the case, as addition of annexin 12 to the trans side of the bilayer resulted in a significant and concentration-dependent decrease in the lateral diffusion of RyR2 channels. The highest concentration of annexin 12 tested extensively, 2 μM, reduced RyR2 diffusion over the 1-min measurement period from domains of ∼±30 μm imaged by ∼±125 pixels in the absence of annexin 12, to domains of ∼±0.36 μm imaged by ∼±3.0 pixels in the presence of annexin 12 (Fig. 2). Thus, it is likely that RyR2 movement is restricted to an area less than that imaged by 1 pixel during measurement intervals of a few seconds or less. This is equivalent to immobilization of RyR2 in our imaging system. As noted previously, the effects of 2 μM annexin 12 on RyR2 diffusion appear to be maximal under our experimental conditions, since similar results were obtained in two experiments with a 4-μM concentration of this agent.
We have not tested whether annexin 12 added to the cis side of the bilayer is able to decrease RyR2 diffusion. Two features of our experimental system dictated that annexin 12 be added to the trans chamber. First, the Ca2+ dependence of the binding of annexin 12 to PS requires micro- to millimolar concentrations of Ca2+. Such Ca2+ levels are only present in the trans chamber. Second, the volume of the trans chamber is much smaller than that of the cis chamber and, thus, it is much more economical to add annexin 12 to the trans side of the bilayer.
Does annexin 12 alter RyR2 channel properties or bilayer conductance?
Use of annexin 12 to immobilize RyR2 during optical analysis of their ion channel properties will be complicated if these properties are altered by annexin 12. Data we have obtained to date for activation of RyR2 channels by suramin and Ca2+ have not revealed any noticeable changes in RyR2 channel behavior in the presence of annexin 12. Importantly, the lack of effect on activation of RyR2 channels by suramin permits use of the voltage command protocol in combination with annexin 12 for analysis of fluorescence responses to Ca2+ flux through single RyR2 channels as described in the preceding report (Peng et al., 2004). Even if minor effects by annexin 12 should be found in the future, a strength of the voltage command protocol is that Ca2+ currents are measured and changes in Ca2+ flux are taken into account. Influences by annexin 12 on RyR2 channel properties will be more of a concern when used in conjunction with optical imaging to investigate relationships between RyR2 structure and channel function in future studies. In this case it will be necessary to demonstrate a lack of effect by annexin 12 in every experimental paradigm studied. Sokolov et al. (2000) observed that different native annexins, as well as mutants of annexin 12, differ in their ability to alter gating kinetics of alamethicin channels. Such differences suggest it may be possible to find a suitable substitute annexin should annexin 12 be found to alter RyR2 channel function.
Another member of the annexin superfamily, annexin 6, has been found to increase the open probability and mean open time of skeletal muscle RyR1 isoform channels reconstituted into lipid bilayers (Diaz-Munoz et al., 1990). To our knowledge, annexin 6 has not been tested directly on RyR2 channels. However, data from annexin 6 null mice and from mice overexpressing annexin 6 indicate the absence of effects on sarcoplasmic reticulum Ca2+ release in heart (Gunteski-Hamblin et al., 1996; Song et al., 2002). We have not yet tested the ability of annexin 6 to alter movement of RyR2 channels in bilayers.
It is of interest to consider why we failed to observe changes in RyR2 channel function in the presence of annexin 12, whereas Sokolov et al. (2000) found that the conductance properties of nonactin and alamethicin channels were altered in the presence of this protein. We think this may be explained by the fact that nonactin and alamethicin are small peptides, which must incorporate into the bilayer and aggregate to form competent channels in a bilayer and annexins influence peptide aggregation, rather than channel activity, per se. RyR2 channels, at least to the extent tested to date, are unaffected by annexin 12, once they have become incorporated in the bilayer. Although we have not systematically characterized the effect of annexin 12 on fusion of RyR2-liposomes with bilayers, it is our opinion that it becomes increasingly difficult to obtain fusion as the concentration of annexin 12 is increased. Although anecdotal, this observation is consistent with an ability of annexin 12 to stiffen and decrease the fluidity of bilayers and retard incorporation of peptides and polypeptides into bilayers.
Annexin 12 has been observed to form ion channels in bilayers under mildly acidic conditions (Isas et al., 2000). The presence of an additional conductance would confound quantitative studies of Ca2+ flux through RyR2 channels. Thus it was important to determine whether the presence of annexins altered bilayer conductance. In this regard, under the conditions used in our experiments, which involved a neutral pH, bilayer conductance was identical in the absence and presence of annexin 12 both before and after incorporation of RyR2 channels. This is consistent with observations by Isas et al. (2000) that annexin 12 inserts and forms ion channels in bilayers in the presence of acidic pH, but only associates with the surface of bilayers when the pH is neutral, as it was in the present study.
How does annexin 12 decrease RyR2 diffusion?
An understanding of the mechanism(s) underlying its effect on RyR2 diffusion will enhance use of annexin 12 as a tool to prevent lateral diffusion of RyR2 in bilayers. Although additional studies are needed to establish the mechanism of action of annexin 12, we propose the following model based on the results described in this report and on data described in the literature.
The physiological functions of annexin 12 and of other annexins are poorly understood. Perhaps the best-characterized aspect of annexin function is the ability of these proteins to associate with biological membranes and lipid bilayers through Ca2+-dependent binding to anionic phospholipids, such as PS (Gerke and Moss, 2002; Swairjo et al., 1994; Swairjo and Seaton, 1994). In keeping with the principle of simplicity, we consider the possibility that annexin 12 prevents RyR2 diffusion solely by binding to PS without invoking direct interaction with RyR2. This can be envisioned as formation of diffusion barriers that restrict movement of RyR2 in the bilayer. In this model, annexin 12 forms an assembly of trimers on the surface of bilayers that interact with PS (Langen et al., 1998). Coordination of PS molecules by annexin 12 creates regions of diminished fluidity that form barriers to diffusion by integral membrane proteins, such as RyR2. The size of bilayer regions delimited by trimer assemblies would decrease as the concentration of annexin 12 is increased and thereby constrain RyR2 to smaller and smaller domains. It is likely that these effects would also diminish convectional flow in bilayers and related RyR2 channel movement.
This diffusion barrier model leads to several predictions, which will be tested in future studies. First, effects of annexin 12 on RyR2 diffusion should require the presence of an anionic phospholipid in the bilayer and sufficient Ca2+ in the trans chamber to ensure binding of annexin 12 with anionic phospholipids. Second, the dependence on annexin 12 concentration should be related primarily to the molar ratio of annexin to anionic phospholipid and not to the absolute concentration of annexin. Third, annexin 12 and other annexins capable of coordinating anionic phospholipid molecules should diminish diffusion of any integral membrane protein reconstituted in a bilayer, as well as that of component phospholipids. In this regard, not all annexins form trimers as observed for annexin 12; however, all annexins bind to multiple anionic phospholipid molecules, and in doing so, may coordinate lipid domains in bilayers. Consequently, all annexins have the potential to decrease diffusion by integral membrane proteins. Consistent with this possibility, we have observed that annexin 2, which does not form trimers (Haigler et al., unpublished observations), is as effective as annexin 12 in decreasing diffusion of RyR2 channels (Peng et al., unpublished observations). Fourth, annexins should not bind to RyR2 reconstituted in the bilayer in a specific manner and should not alter the function of this protein.
CONCLUSIONS
We have used fluorescence imaging to characterize lateral diffusion of single RyR2 channels in planar lipid bilayers. We have found that addition of micromolar concentrations of annexin 12 to the trans side of the bilayer markedly decreases RyR2 diffusion and essentially immobilizes RyR2 to domains smaller than those imaged by a single pixel during intervals of a few seconds. Importantly, we have not observed any effects by annexin 12 on bilayer conductance or on the ability of either suramin or Ca2+ to activate RyR2 channels. Thus, annexin 12 and perhaps other annexins have the potential to serve as an important tool for limiting lateral diffusion of RyR2 channels and thereby optimizing the spatial and temporal resolution of optical imaging techniques used to analyze the ion channel properties of this protein.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Acknowledgments
We thank Alan Williams and Rebecca Sitsapesan for critiquing a draft of this report.
The authors received support from the following sources: S.P., J.A.A., and J.L.S. from National Institutes of Health grants HL53677 and AR45112; N.G.P. and J.L.S. from a National Science Foundation EPSCoR grant EPS0132556; H.H. from National Institutes of Health grant GM55651; and J.E.H. from National Institutes of Health grant GM57998.
References
- Diaz-Munoz, M., S. L. Hamilton, M. A. Kaetzel, P. Hazarika, and J. R. Dedman. 1990. Modulation of Ca2+ release channel activity from sarcoplasmic reticulum by annexin VI (67-kDa calcimedin). J. Biol. Chem. 265:15894–15899. [PubMed] [Google Scholar]
- Gerke, V., and S. E. Moss. 2002. Annexins: from structure to function. Physiol. Rev. 82:331–371. [DOI] [PubMed] [Google Scholar]
- Gunteski-Hamblin, A. M., G. Song, R. A. Walsh, M. Frenzke, G. P. Boivin, G. W. Dorn 2nd, M A. Kaetzel, N. D. Horseman, and J. R. Dedman. 1996. Annexin VI overexpression targeted to heart alters cardiomyocyte function in transgenic mice. Am. J. Physiol. 270:H1091–H1100. [DOI] [PubMed] [Google Scholar]
- Isas, J. M., J. P. Cartailler, Y. Sokolov, D. R. Patel, R. Langen, H. Luecke, J. E. Hall, and H. T. Haigler. 2000. Annexins V and XII insert into bilayers at mildly acidic pH and form ion channels. Biochemistry. 39:3015–3022. [DOI] [PubMed] [Google Scholar]
- Langen, R., J. M. Isas, H. Luecke, H. T. Haigler, and W. L. Hubbell. 1998. Membrane-mediated assembly of annexins studied by site-directed spin labeling. J. Biol. Chem. 273:22453–22457. [DOI] [PubMed] [Google Scholar]
- Liu, Z., J. Zhang, P. Li, S. R. Chen, and T. Wagenknecht. 2002. Three-dimensional reconstruction of the recombinant type 2 ryanodine receptor and localization of its divergent region 1. J. Biol. Chem. 277:46712–46719. [DOI] [PubMed] [Google Scholar]
- Patel, D. R., C. C. Jao, W. S. Mailliard, J. M. Isas, R. Langen, and H. T. Haigler. 2001. Calcium-dependent binding of annexin 12 to phospholipid bilayers: stoichiometry and implications. Biochemistry. 40:7054–7060. [DOI] [PubMed] [Google Scholar]
- Peng, S., N. G. Publicover, G. J. Kargacin, D. Duan, J. A. Airey, and J. L. Sutko. 2004. Imaging single cardiac ryanodine receptor Ca2+ fluxes in lipid bilayers. Biophys. J. 86:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, S., N. Publicover, J. Airey, H. Haigler, J. Hall, J. Zhang, S. R. W. Chen, and J. Sutko. 2003b. Immobilization of ryanodine receptor (RyR) channels in bilayers for high fidelity optical recording. Biophys J. 84:387a. (Abstr.) [Google Scholar]
- Qian, H., M. P. Sheetz, and E. L. Elson. 1991. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys. J. 60:910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov, Y., W. S. Mailliard, N. Tranngo, M. Isas, H. Luecke, H. T. Haigler, and J. E. Hall. 2000. Annexins V and XII alter the properties of planar lipid bilayers seen by conductance probes. J. Gen. Physiol. 115:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, G., S. E. Harding, M. R. Duchen, R. Tunwell, P. O'Gara, T. E. Hawkins, and S. E. Moss. 2002. Altered mechanical properties and intracellular calcium signaling in cardiomyocytes from annexin 6 null-mutant mice. FASEB J. 16:622–624. [DOI] [PubMed] [Google Scholar]
- Swairjo, M. A., M. F. Roberts, M. B. Campos, J. R. Dedman, and B. A. Seaton. 1994. Annexin V binding to the outer leaflet of small unilamellar vesicles leads to altered inner-leaflet properties: 31P- and 1H-NMR studies. Biochemistry. 33:10944–10950. [DOI] [PubMed] [Google Scholar]
- Swairjo, M. A., and B. A. Seaton. 1994. Annexin structure and membrane interactions: a molecular perspective. Annu. Rev. Biophys. Biomol. Struct. 23:193–213. [DOI] [PubMed] [Google Scholar]
- Wagner, M. L., and L. K. Tamm. 2001. Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys. J. 81:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]