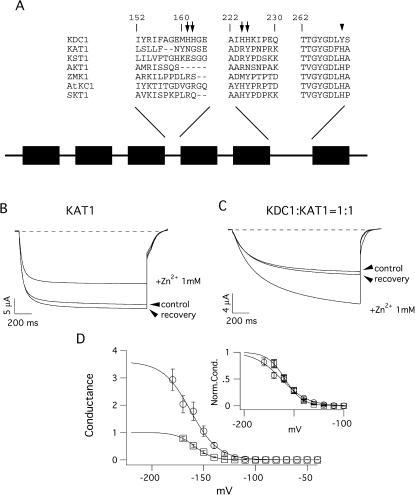
FIGURE 1.
KDC1 and KAT1 schematic structure and effects of zinc on the heteromeric KDC1:KAT1 channel. (A) Segment sequences comprising KDC1 histidines compared with the correspondent segments in other plant inward-rectifying potassium channels. All channels belong to the Shaker family and therefore comprise six hydrophobic transmembrane segments. The S4 segment is the voltage sensor motif and the putative pore region is located between the S5 and S6 segments. The locations of the KDC1 histidines are indicated by arrows in the segments comprised between S3 and S4 and S5 and S6. Instead KAT1 has only one histidine residue in the pore region (H267), whereas in the correspondent position KDC1 has a tyrosine (Y269), indicated by the arrowhead. (B) The addition of 1 mM extracellular Zn2+ typically inhibited up to 30–40% of the KAT1 current. (C) Conversely, with the addition of 1 mM Zn2+, the heteromeric KDC1:KAT1 channels displayed a marked increase in the current. Applied transmembrane potentials to −150 mV. (D) Increase of the macroscopic conductance in the KDC1:KAT1 channel after addition of 5 mM zinc (unfilled circles) compared with the conductance of the same channel in the absence of zinc (unfilled squares, control). (Inset) Comparison of the normalized conductances obtained in the presence and absence of zinc. The symbols have the same meaning as in the panel. Each data point was obtained from three to eight experiments. Standard ionic solution, pH 5.6.