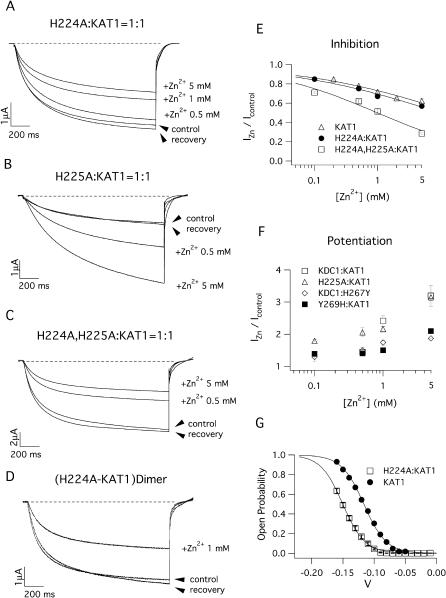
FIGURE 5.
Sensitivity to external Zn2+ of heteromeric channels comprising KDC1 mutated in the S5–S6 histidines. Ionic currents recorded in the presence of Zn2+ in the bath solution from oocytes injected with mRNA mixtures containing (A) H224A:KAT1, (B) H225A:KAT1, and (C) (H224A,H225A):KAT1. Holding, step, and tail potentials were 0 mV, −150 mV, and −70 mV, respectively. (D) 1 mM Zn2+ reversibly inhibits the current mediated by the dimeric mutated channel H224A-KAT1. Compare these effects with those induced on the heteromultimeric channel shown in A. (E) Dose-response inhibition for KAT1 and mutated KDC1 (H224A and H224A,H225A): KAT1 heteromeric channels. Continuous lines are the best fit of the equation IZn/Icont = Kin/(Kin + xn) where Ki is the zinc concentration that inhibits one-half of the maximal current and n is the Hill coefficient. We found Ki = 15 mM for KAT1 (n = 0.4), Ki = 9.7 mM for H224A:KAT1 (n = 0.4), and Ki = 0.98 mM for H224A,H225A:KAT1 (n = 0.5). (F) Dose-response curves of current potentiation for KDC1 and mutated KDC1 (H225A and Y269H) as well as mutated KAT1 (H267Y). Data represent mean ± SE from at least three experiments. Applied transmembrane potentials to −150 mV. (G) Comparison of the normalized conductances of KAT1 and H224A:KAT1 heteromultimeric channels; the significant shift of the opening probability induced by KDC1 on KAT1 characteristics can be observed. Standard ionic solution, pH 5.6.