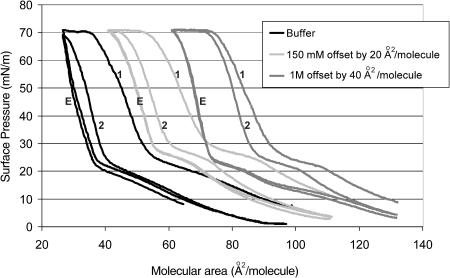
FIGURE 1.
First compression (1); expansion (E); second compression (2). Langmuir isotherms (offset by 20 A2/mol) of dipalmitoylphosphatidylglycerol at pH 7 at 30°C, well above the triple point for DPPG. The isotherm to the left is for a DPPG monolayer on a subphase of 0.2 mM sodium bicarbonate, the center isotherm is for DPPG on 0.2 mM sodium bicarbonate with 150 mM sodium chloride, and the isotherm to the right is for DPPG on 0.2 mM sodium bicarbonate with 1 M NaCl. The same amount of DPPG was spread from a chloroform solution for each isotherm. The main difference between the isotherms is the second compression cycle (labeled 2). For the 1-M isotherm, the second compression almost retraces the first (labeled 1), indicating little material lost and the fractional recovery is 0.95. For the isotherm on buffer only, the second compression is shifted toward smaller areas per molecule in comparison to the first compression, indicating a significant loss of material from the monolayer. The fractional recovery was only ∼0.30. The expansion isotherms were nearly identical, suggesting that the surface pressure at which material was reincorporated (plateau at ∼25 mN/m) was independent of the ionic strength.