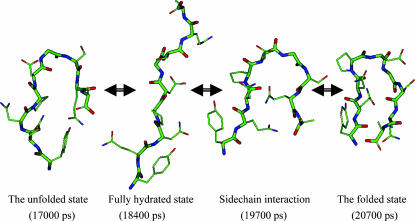
FIGURE 10.
Typical conformational changes during a β-hairpin folding. The conformation at 17,000 ps represents an unfolded structure with some intrapeptide interactions. At 184,000 ps the peptide becomes fully hydrated. Side-chain interactions bring the two strands together at 19,700 ps. At 20,700 ps the peptide reaches a β-hairpin structure. Only heavy atoms are shown. Backbone atoms are shown as thick sticks. Atoms are colored as described in the legend of Fig. 2.