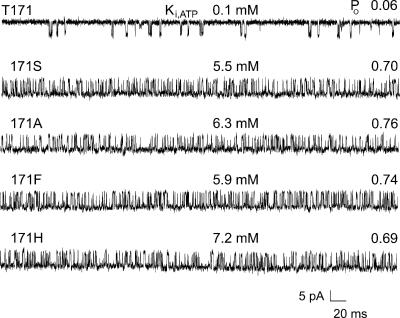
FIGURE 1.
Substitutions at T171 dramatically slow gating from the active burst to the inactive interburst in truncated Kir6.2 channels without SUR1. Of all possible substitutions constructed only four, S, A, F, and H, allowed detectable channel expression. Each of these substitutions substantially increased Ki,ATP by 55-fold or more and importantly increased PO by 11-fold or more. Representative single-channel records are shown with the single-letter abbreviation for the amino acid residue at position 171 of Kir6.2 indicated. The top trace is from a channel with the wild-type T at position 171. These control channels had a relatively low Ki for ATP inhibition of 0.1 mM and a low PO in the absence of ATP of 0.06. For both Ki,ATP and PO values of each channel, n ≥ 5, with mutant values significantly different from wild-type values, P < 0.001.