FIGURE 7.
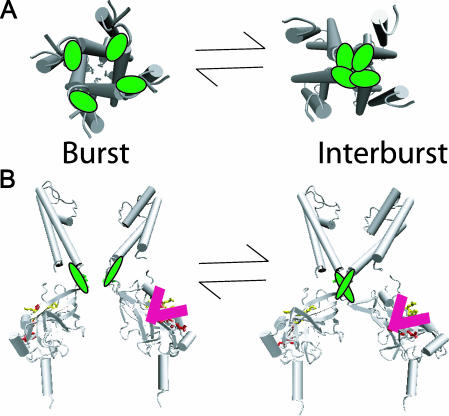
Concerted action of all four Kir6.2 subunits underlies burst gate closure. (A) The view from the cytoplasm depicts one-step occlusion of the pore. The gating results from highly cooperative tertiary conformational changes involving T171 determinants (green) at the cytoplasmic end of the inner helix of each subunit. (B) The membrane cross sectional view depicts the topological relation between T171 residues (green) and the candidate inhibitory ATP site residues I182, K185, R201, and G334 (red). Only two subunits are shown in the panel for clarity. Occupancy of one of the ATP sites of the cytoplasmic C-terminal domain of a single Kir6.2 subunit can cause concerted conformational changes in all four Kir6.2 subunits, to shut the intracellular M2 burst gate. Conformational changes accompanying ATP binding to any one of the four subunits are independent, and yet each independent conformational change is coupled to a concerted conformational change in which all four subunits act to close the intracellular M2 burst gate. The closed gating conformation of each subunit, however, does not couple to its site unless it is occupied by ATP. This ligand-dependent linkage, coupling conformations of occupied ATP site and closed intracellular M2 burst gate, loosens the constraint that conformational changes at the gate, and conformational changes at the site, always exhibit tight reciprocity, which explains much of what is known about ATP-dependent inhibition gating of the KATP channel.