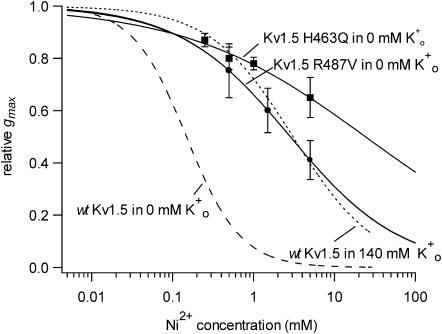
FIGURE 5.
Ni2+ sensitivity is reduced in Kv1.5 H463Q and Kv1.5 R487V. Experiments with the mutant channels were done with 0 mM Ko+ to preclude a change of the Ko+ binding as the basis for the change of the sensitivity to Ni2+. Because Ni2+ is known to bind to histidine (H) residues, a mutant was constructed in which glutamine (Q) was substituted for H463, a residue in the S5-P linker that forms part of the outer pore vestibule. Kv1.5 H463Q (▪) was from 100- to 200-fold less sensitive to Ni2+ (KD = 24 ± 8 mM; nH = 0.4 ± 0.04) compared to the wt Kv1.5 responses (dashed line taken from Fig. 2) measured in the same recording condition, i.e., 0 mM Ko+. In another mutant construct, the arginine (R) residue near the entrance to the pore mouth that has been implicated, by alignment with Shaker T449, in the outer pore inactivation mechanism, was mutated to valine. The sensitivity of Kv1.5 R487V currents (•) to Ni2+ was ∼20-fold less (KD = 2.8 ± 0.004 mM; nH = 0.7 ± 0.001) than that measured under the same recording conditions in wt Kv1.5. The dotted line, which was taken from Fig. 2, represents the line fitted to the block of wt Kv1.5 in 140 mM Ko+.