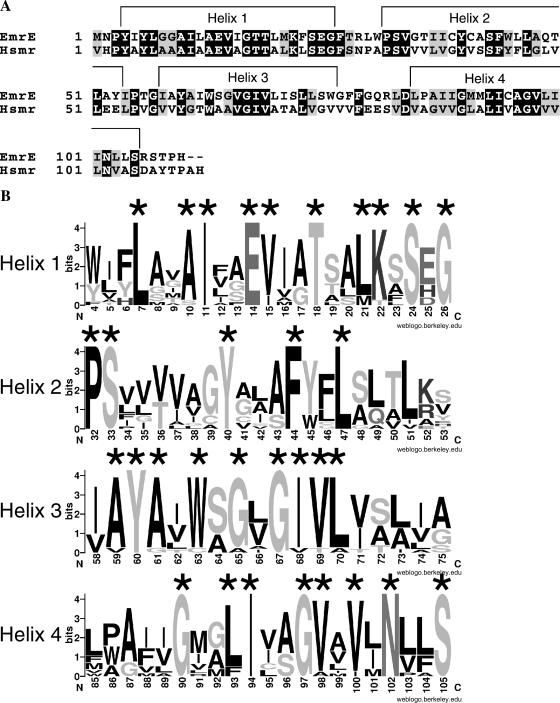
FIGURE 1.
Sequences and secondary structure of EmrE and Hsmr. (A) The sequences of EmrE and Hsmr are shown, with identical residues having a black background and similar residues a gray background. The shown secondary structure has been determined for EmrE using high-resolution NMR experiments. (B) Information content of a multiple alignment of 14 highly homologous sequences including EmrE and Hsmr is shown as sequence logos, generated with the web-based application WebLogo (http://weblogo.berkeley.edu). The overall height of each logo indicates the conservation at this position, whereas the height of each individual residue indicates the frequency of occurrence of this particular amino acid at this position. The topmost amino acid at each position corresponds to the consensus sequence.