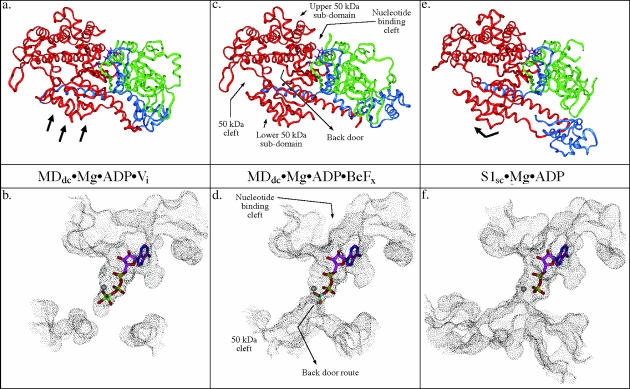
FIGURE 1.
Comparison of solvent-accessible surface representations of the nucleotide-binding and back door region of myosin in three different conformations. This figure shows relative states of the back door in the three crystal structure observed conformations of myosin and the associated subdomain movements within the motor domain. Also shown is the position of the nucleotide/nucleotide analog. The MDdc·Mg·ADP·Vi crystal structure (a and b) (Smith and Rayment, 1996b) shows that the solvent accessible surface is discontinuous between the phosphate tube and the 50-kDa cleft indicating a closed back door state. The MDdc·Mg·ADP·BeFx crystal structure (c and d) (Fisher et al., 1995) shows the solvent-accessible surface extending all the way from the nucleotide-binding pocket and phosphate tube into the 50-kDa cleft revealing a partially open back door state. The S1sc·Mg·ADP crystal structure (e and f) (Houdusse et al., 1999) shows a 50-kDa cleft and back door region that appears even more open than the MDdc·Mg·ADP·BeFx structure (note the increased size of the accessible surface in the 50-kDa cleft relative to the rigor state). In a, c, and e, the 25-kDa tryptic fragment is colored green, the 50-kDa fragment is red, and the 20-kDa fragment is blue. The black arrows in a and e indicate the repositioning of the lower 50-kDa subdomain relative to the MDdc·Mg·ADP·BeFx structure in c.