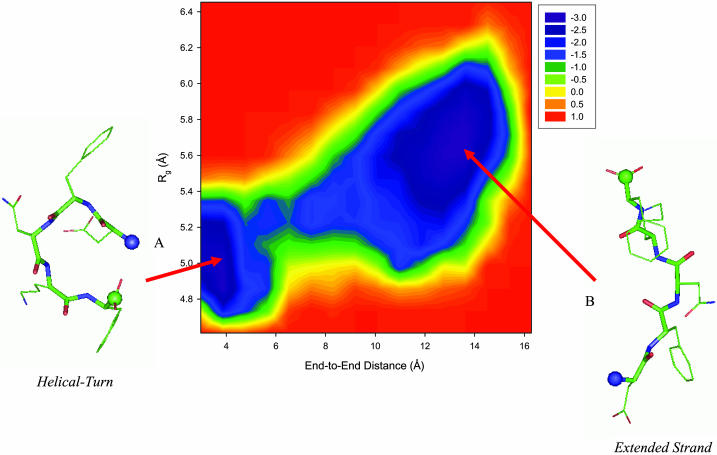
FIGURE 3.
Free energy profile of the DFNKF monomer as a function of the end-to-end distance and the radius of gyration at 350 K. The energy scale is in RT instead of kcal/mol. Two main basins (minima, shown in purple) are identified. (A) Helical turn-like conformation. This structure has a shorter end-to-end distance and a smaller radius of gyration with higher helical propensity. (B) Extended β-strand-like conformation. The conformation of this state is extended with an end-to-end distance of ∼13 Å. Secondary structure analysis based on the ψ- and φ-angles shows that the conformations within this basin have higher β-strand content. Representative structures of these two basins are shown along with the free energy landscape. The backbone atoms are shown as thick sticks, whereas the side-chain atoms are presented as thin sticks. The N- and C-termini are denoted by blue and green balls, respectively.