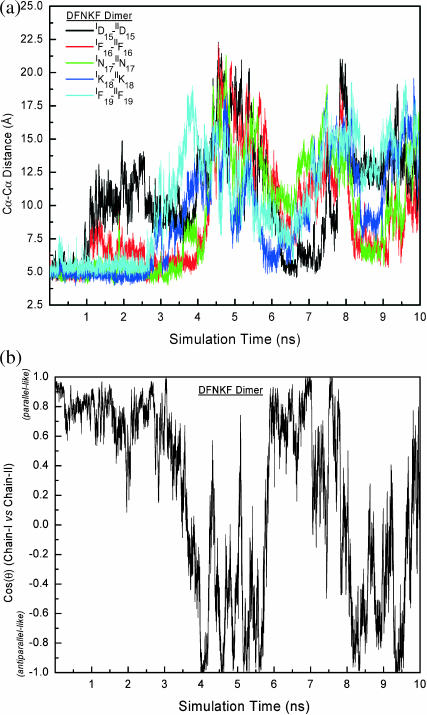
FIGURE 4.
Structural characteristics (pairwise residue Cα-Cα distances and cross angle between chains) of the DFNKF dimer. (a) The pairwise residue Cα-Cα distances change with time. The Cα-Cα distances are calculated based on Cα atom distances between a residue pair. The charged (Asp and Lys) and C-terminus residues (IF19 and IIF19) are more flexible at the early events of the simulation. The five pair-residue distances plotted here reach the maximum roughly at the same time (t ∼ 5 ns) and return to their minima together at ∼6.5 ns showing that some periodic events occur during the simulation. (b) The time dependence of cos(θ), the cross angle between chain-I and chain-II. The definition of cos(θ) is described in Computational Methods. The oscillation between parallel and antiparallel arrangement is observed.