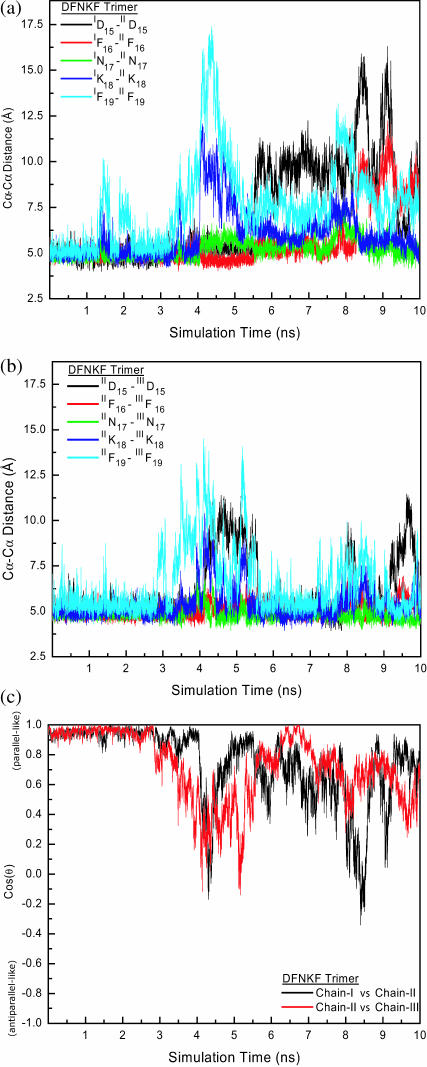
FIGURE 6.
Structural characteristics (pairwise residue Cα-Cα distances and cross angle between chains) of the DFNKF trimer. (a) The pairwise residue Cα-Cα distances between chain-I and chain-II. (b) The pairwise residue Cα-Cα distances between chain-II and chain-III. (c). The time dependence of cos(θ), the cross angle between the chains. The definition of cos(θ) is given in Computational Methods. Only the cross angles between chain-I versus chain-II and chain-II versus chain-III are shown. The DFNKF trimer stays at the in-register parallel structure until ∼2.5 ns. After 2.5 ns, the structural characteristics between chain-I and chain-II lose their parallel structural character. In contrast, the structure between chain-II and chain-III is in parallel during the simulation time between ∼5.5 ns and ∼7.0 ns. Chain-II, whose conformational space is restrained by chain-I and chain-II, has less conformational entropy, providing a β-sheet template for the chain-III registration. In general, the charged and C-terminal residues are more flexible.