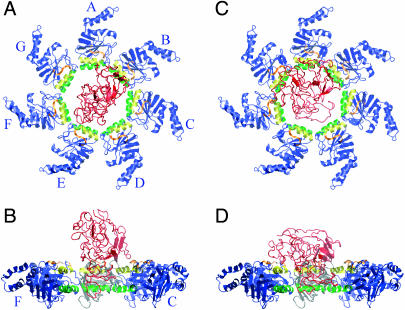
FIGURE 1.
Snapshots of the initial configurations of rhodanese. A and B show the starting configuration for the study of denatured rhodanese and closed-state GroEL; there are no contacts between GroEL and rhodanese in this configuration. C and D show the starting configurations for the TMD simulation; these are the endpoints of the closed-state simulation. The overall motion of rhodanese during the closed-state simulation is a translation by 10.7 Å and a 45.9° rotation. The H helices of GroEL are colored yellow, the I helices are green, the loop formed by residues 310–315 is orange, and the rest of the GroEL apical domains are blue. Rhodanese is shown in red. A and C show the top views, in which the A subunit is at the 12-o'clock position and the other subunits (B–G) follow in a clockwise fashion as indicated. B and D show the side views, obtained by a 90° rotation. For clarity, subunits D and E have been removed in B and D, and subunit A is colored gray. Figs. 1, 2, and 6–8 were prepared with VMD (Humphrey et al., 1996).