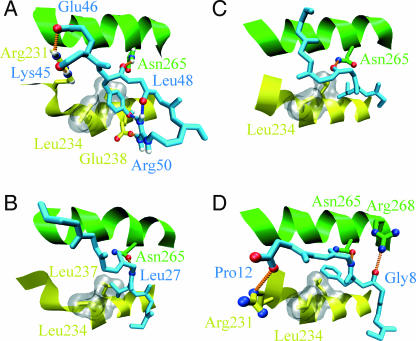
FIGURE 6.
Binding to closed-state GroEL in simulation and experiments. The peptides are shown in light blue, the GroEL H helix is yellow, and the GroEL I helix is shown in green. The hydrophobic pocket formed by Leu-234 and Leu-237 of the H helix is shown by the gray surface. Hydrogen bonds between the peptide and GroEL are indicated by the orange dotted lines; intramolecular hydrogen bonds in the peptides are indicated by blue dotted lines. (A) The starting TMD configuration for rhodanese residues 45–50. Hydrogen bonds are formed between rhodanese Lys-45 and GroEL Arg-231, rhodanese Glu-46 and GroEL Arg-231, rhodanese Tyr-47 and Asn-265, and rhodanese Arg-50 and GroEL Glu-238. Intramolecular hydrogen bonds exist between rhodanese Tyr-47 and Arg-50 and between rhodanese Leu-48 and Arg-50. For clarity, the hydrogen bond between Glu-46 and Arg-268 of the E subunit is excluded from the figure. The hydrophobic pocket is occupied by Tyr-47. (B) GroES residues 24–30 binding to GroEL (Xu et al., 1997). There is a hydrogen bond between GroES Leu-27 and GroEL Asn-265; the hydrophobic pocket is occupied by Val-26. (C) Residues 184-189 of the N-terminal extension binding to GroEL (Buckle et al., 1997). There is a hydrogen bond between Val-186 of the N-terminal extension and Asn-265 of GroEL. The hydrophobic pocket is occupied by Leu-185. (D) Residues 6–12 of the SBP peptide binding to GroEL, taken from chain F and B of the protein data bank structure 1DKD (Chen and Sigler, 1999). Hydrogen bonds are formed between SBP Gly8 and GroEL Arg-268, SBP Leu-10, and GroEL Asn-265, and SBP Pro-12 and GroEL Arg-231. The hydrophobic pocket is occupied by Phe-9.