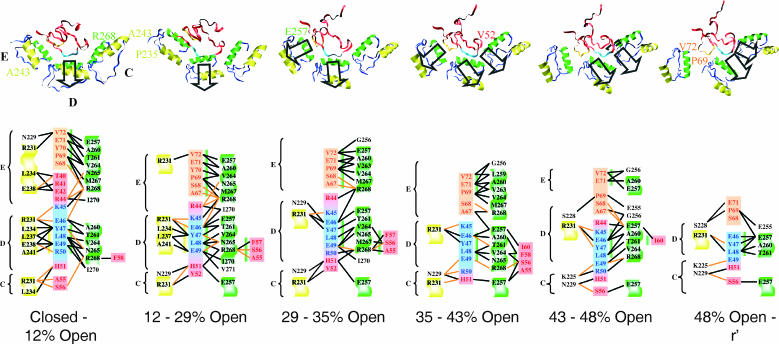
FIGURE 7.
Stretching and interactions for the 42–70 loop of rhodanese. The H helices of GroEL (residues 234–243) are colored yellow, the I helices (residues 257–268) are green, and the rest of GroEL is blue. The symbols C, D, and E refer to the different GroEL subunits. GroEL is viewed from the top of subunit D, looking down into the cis cavity. The viewing angle is the same for all snapshots. Rhodanese residues 45–50 are light blue, residues 67–72 are orange; the other rhodanese residues are shown in red. The direction of the stretching force is indicated by the black arrows. The diagrams below show the interactions between these rhodanese residues and GroEL during part of the transition. Hydrogen bonding is indicated by the orange lines, heavy atom contacts within 4.0 Å, which are mainly van der Waals contacts, are shown by the black lines. The diagrams show all interactions that are present for 10 ps or more during the entire interval; not all contacts and hydrogen bonds are present at every instant of the interval. In the diagrams the H helix is shown on a yellow background, the I helix on a green background, and other GroEL residues are shown on a white background. Residue Arg-231 of GroEL is shown on a yellow background to indicate the closeness of this residue to the H helix. The coloring of rhodanese is identical to the structures above. Binding of rhodanese parallel to the H helix is indicated by a yellow bar (left), binding of rhodanese parallel to the I helix by the green bar (right and left). The presence of both bars for certain residues (e.g., Glu-46, Tyr-47) indicate that this residue was bound in the cleft formed by the H and I helices; this happens in the closed to 12% open and the 12% to 29% open intervals.