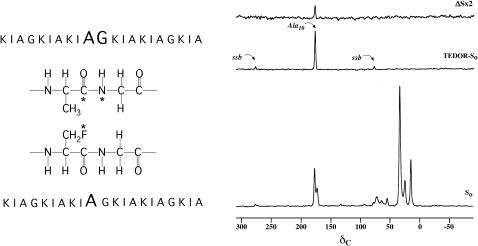
FIGURE 1.
(Left) Positions of the 13C, 15N, and 19F labels in the two K3 analogs ([1-13C]Ala10-[15N]Gly11-(KIAGKIA)3-NH2 and [2-2H, 3-19F]Ala10-(KIAGKIA)3-NH2) used in the REDOR experiments to detect aggregation of peptide chains. (Right) 50.3-MHz 15N → 13C{19F} TEDOR-REDOR NMR spectra of a 50-50 mixture KIA)3-NH2 and [2-2H, 3-19F]Ala10-(KIAGKIA)3-NH2 incorporated into synthetic MLVs of DPPG/DPPC (1:1) at a lipid/peptide molar ratio of 20, after 48 rotor cycles of dipolar evolution with magic-angle spinning at 5000 Hz. The 48-Tr full-echo spectrum (So) is shown at the bottom of the figure. The middle spectrum resulted from a 4-Tr 15N → 13C TEDOR coherence transfer followed by 48 additional rotor cycles with high power proton decoupling. The 48-Tr REDOR ΔS spectrum of the TEDOR-selected signal is shown at the top of the figure.