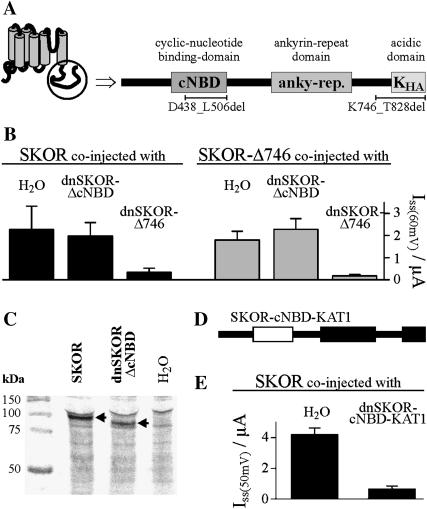
FIGURE 3.
The putative cyclic-nucleotide binding domain, but not the acidic domain, is essential for SKOR assembly. (A) Schematic representation of the cytoplasmic SKOR C-terminus. The C-terminus contains three regions showing strong homology to previously characterized functional domains: a cyclic-nucleotide binding domain (cNBD), an ankyrin domain (anky-rep.), and an acidic domain (KHA). (B) K+ current amplitudes (ISS) measured in oocytes after coexpression (injection of 1:1 cRNA mixtures) of SKOR (solid columns) and SKOR-Δ746 (= SKOR-K746X-E747_T828del, shaded columns), respectively, with dominant-negative mutants of SKOR-Δ746 (dnSKOR-Δ746 = SKOR-G292R-D293N-A296V-K746X-E747_T828del, right columns) and SKOR-ΔcNBD (dnSKOR-ΔcNBD = SKOR-G292R-D293N-A296V-D438_L506del, middle columns), and H2O (left columns). Current amplitudes were measured at the end of 3-s voltage pulses to +60 mV. Data are shown as means ± SD (n = 3–6). (C) The protein dnSKOR-ΔcNBD is expressed in Xenopus oocytes. 35S-labeled proteins obtained from water-injected control oocytes (right lane) and oocytes injected with SKOR or dnSKOR-ΔcNBD cRNA, respectively (left and middle lanes). Proteins were analyzed by SDS-PAGE followed by autoradiography. Molecular weight markers are on the left. Calculated molecular weights are 93.9 kDa for SKOR, and 86.1 kDa for dnSKOR-ΔcNBD. Arrows indicate the heterologously expressed proteins. (D) C-terminus of the chimera dnSKOR-cNBD-KAT1. The cNBD of SKOR (fragment SKOR-E410_L530) was replaced by the cNBD of KAT1 (fragment KAT1-N384_L493, white rectangle). (E) K+ current amplitudes (ISS) measured in oocytes after coexpression (injection of 1:1 cRNA mixtures) of SKOR with the dominant-negative mutant dnSKOR-cNBD-KAT1 (right column) or H2O (left column). Current amplitudes were measured at the end of 3-s voltage pulses to +50 mV. Data are shown as means ± SD (n = 5–6).