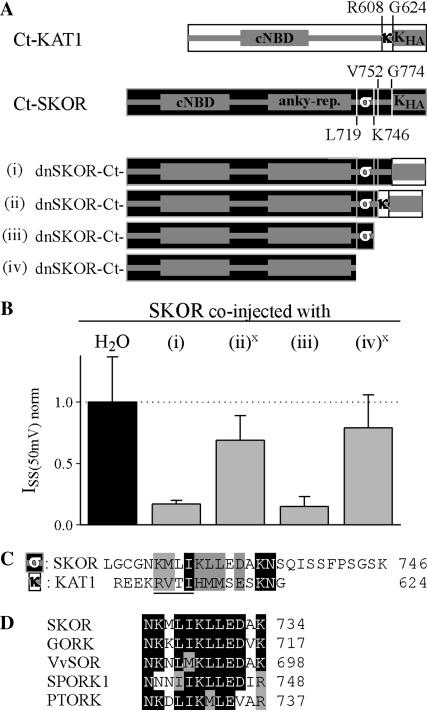
FIGURE 6.
A second domain at the C-terminus is involved in SKOR channel assembly. (A) Generation of chimeric/truncation mutants on the basis of dnSKOR. In dnSKOR, regions at the end of the C-terminus were either deleted or replaced by equivalent parts of KAT1. The individual C-termini are represented schematically (i–iv). The position of the first amino acid of each region is indicated. Parts originating from KAT1 are illustrated by white rectangles, and parts from SKOR are shown as black rectangles. (B) Coexpression experiments (1:1 cRNA mixtures) with SKOR and the constructs shown in panel A in Xenopus oocytes. Current amplitudes (ISS) were measured at the end of 2-s voltage pulses to +50 mV. To compare results obtained from different oocyte batches, the data were normalized to the mean value of the control SKOR/H2O (solid column). Data are shown as means ± SD (n = 6–12, two different oocyte batches). The expression of the constructs (ii) and (iv) (marked with x) was verified in 35S-protein-labeling experiments. (C) Sequence comparison of the regions “σ” (SKOR-L719_K746) and “κ” (KAT1-R608_G624) marked in A. The conserved tetra-peptide of the KT domain ([R-V-T-I] in KAT1) is underlined. (D) Sequence comparison of the “σ” domains of the plant Kout channels SKOR (GenBank protein accession number CAA11280), GORK (CAC17380), Vitis vinifera VvSOR (CAD35400), Samaea saman SPORK1 (CAC10514), and Populus tremula x tremuloides PTORK (CAC05488).