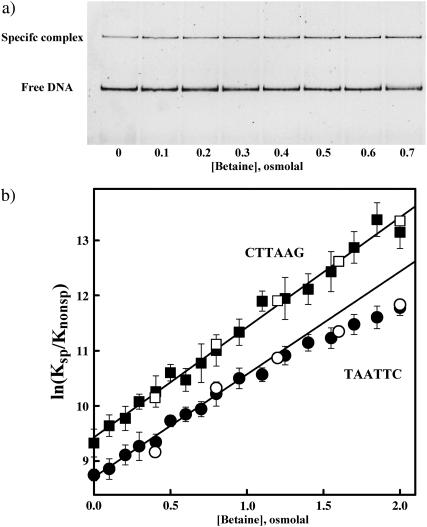
FIGURE 1.
(a) A polyacrylamide gel image illustrating that osmotic pressure favors specific sequence EcoRI binding over nonspecific binding in competition experiments. The gel mobility shift assay is used to monitor EcoRI binding to a 360-bp fragment containing its recognition sequence. The concentrations of protein, specific site fragment, and inverted sequence oligonucleotide competitor are constant as the concentration of the osmolyte betaine glycine is increased. The fraction of specific sequence complex is determined from fluorescent intensities of the SYBR Green I stained DNA bands. The short 30-bp competitor oligonucleotide is not seen on the gel. The particular conditions were: 120 mM NaCl, 20 mM imidazole pH 7.6, 20°C. Competitor oligonucleotide concentration is 14 μM; EcoRI and specific fragment concentrations are 0.75 and 1.5 nM, respectively. (b) The dependence of ln(Ksp/Knonsp) on betaine glycine or methyl glucoside osmolal concentration is shown for two noncognate oligonucleotides. The ratio of association binding constants for specific and nonspecific sequences, Ksp/Knonsp, was calculated from experiments as shown in Fig. 1 a using Eq. 2. The nonspecific competitors are the TAATTC “star” sequence oligonucleotide (•, betaine; ○, methyl glucoside) and the nonspecific CTTAAG inverted sequence oligonucleotide (▪, betaine; □, methyl glucoside). Except for the central six basepairs, the two 30-bp oligonucleotides are otherwise identical; full sequences are given in Methods and Materials. The error bars (shown for betaine) are calculated from three to four experiments at each osmotic pressure. Experimental conditions are given in Methods and Materials. The slopes at low pressures correspond to 105–110 more waters sequestered by the nonspecific sequence complexes compared with the specific site complex. The linear dependence on osmotic pressure for competition with the inverted sequence oligonucleotide indicates there is no change in the number of water sequestered by this nonspecific complex throughout the entire pressure range. The TAATTC sequence complex, however, apparently begins to lose water at osmotic pressures higher than 1–1.5 Osm.