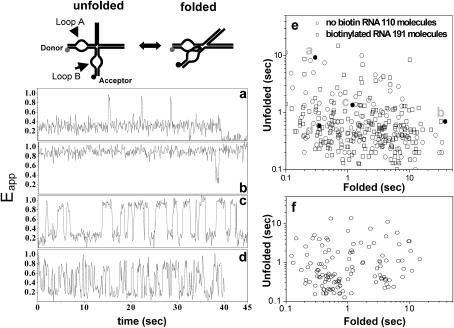
FIGURE 5.
The dynamic properties of hairpin ribozymes as encapsulated species. The ribozymes have been studied in their natural four-way junction form, encapsulated within 200-nm vesicles in 0.5 mM Mg2+ at room temperature. (a–d) Four representative time traces of Eapp clearly show significant variation in the kinetics of folding-unfolding transitions between molecules. (a) This molecule spends most of the time in the unfolded state, but occasionally adopts the folded state for a brief period. (b) In marked contrast, this molecule remains in the folded conformation for most of the time. (c) A molecule undergoing continuous unfolding-folding fluctuations. (d) This molecule also exhibits continuous unfolding-folding transitions, but with shorter dwell times for both the folded and the unfolded states. (e) Scatter plot for the algebraic average of unfolded and folded dwell times for many molecules, calculated from their time traces. The distribution is broad and both the unfolding-folding times exhibit up to 50-fold variance. Encapsulation samples prepared using ribozymes without (circles) and with (squares) biotin are shown on the same plot, and they are similarly distributed. The points corresponding to traces a–d are indicated in the plot (filled circles). (f) Scatter plot for the surface-immobilized molecules adapted from Tan et al. (2003).