TABLE 2.
Thermodynamic constraints on passive and active end-tracking reactions
| Mechanism-A: end-tracking stepping motor | |||
| Steps: | |||
| 1. Monomer binding from solution, ΔG1 = − kT ln([MT]/K1). | |||
| 2. Step of tracking unit to terminal subunit, ΔG2 = kT ln(K2). | |||
| Active motor: Hydrolysis energy ɛ facilitates Step 2. | |||
| Type | Energy | Equilibrium constants | Implications |
| Passive | ΔG1 + ΔG2 = ΔG(+),add ∼ O(−kT) | K1K2 = [MT](+)-crit | Favorable monomer binding ⇒ unfavorable stepping |
| Favorable stepping ⇒ unfavorable monomer binding | |||
| Active | ΔG1 + ΔG2 = ΔG(+),add−ɛ ≪ −kT | K1K2 = [MT](+)-crite−ɛ/kT | Monomer binding and stepping may both be favorable. |
| Mechanism-B: direct-transfer end-tracking motor | |||
| Steps: | |||
1′. Monomer binding from solution, 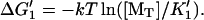
| |||
2′. Transfer of monomer to filament end, 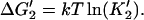
| |||
3′. Release of tracking unit, 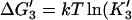 ). ). | |||
| Active motor: Hydrolysis energy ɛ facilitates Step 3 | |||
| Type | Energy | Equilibrium constants | Implications |
| Passive | 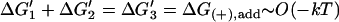 |
K1K2/K3 = [MT](+)-crit | Favorable monomer binding ⇒ unfavorable monomer transfer and/or tracker release |
| Favorable monomer transfer ⇒ unfavorable monomer binding and/or tracker release | |||
| Favorable tracking-unit release ⇒ unfavorable monomer binding and/or monomer transfer | |||
| Active | 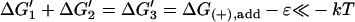 |
K1K2/K3 = [MT](+)-crite−ɛ/kT | Monomer binding, transfer, and tracking unit release may all be favorable. |
