TABLE 2.
Effect of peptide concentration on β-conformation of each residue
| Fraction in β-conformation
|
Folding free energy (kcal/mol)
|
|||||
|---|---|---|---|---|---|---|
| Single low | Single high | Quad | Single low | Single high | Quad | |
| Asn | 0.59 (0.02) | 0.40 (0.01) | 0.65 (0.01) | −0.21 (0.04) | 0.22 (0.03) | −0.35 (0.03) |
| Phe | 0.50 (0.01) | 0.55 (0.01) | 0.67 (0.01) | 0.00 (0.03) | −0.11 (0.03) | −0.38 (0.03) |
| Ala | 0.51 (0.02) | 0.40 (0.01) | 0.63 (0.01) | −0.03 (0.04) | 0.22 (0.03) | −0.29 (0.03) |
| Ile | 0.67 (0.03) | 0.16 (0.02) | 0.55 (0.01) | −0.39 (0.06) | 0.91 (0.04) | −0.12 (0.04) |
| Leu | 0.60 (0.02) | 0.44 (0.03) | 0.55 (0.01) | −0.22 (0.04) | 0.14 (0.06) | −0.11 (0.03) |
| Average | 0.57 (0.02) | 0.39 (0.02) | 0.61 (0.01) | −0.17 (0.04) | 0.25 (0.04) | −0.25 (0.03) |
Free energies were estimated based on 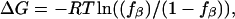 where fβ is the fraction in conformation in the last 5 ns. The Gly residue was excluded from analysis. Standard deviations are shown in parentheses.
where fβ is the fraction in conformation in the last 5 ns. The Gly residue was excluded from analysis. Standard deviations are shown in parentheses.
