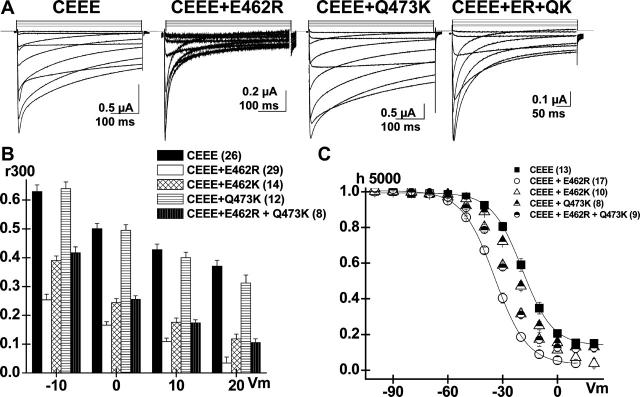
FIGURE 5.
E462R increases the VDI kinetics and voltage dependence of the CEEE chimera. (A) Whole-cell current traces are shown for CEEE mutants: CEEE; CEEE + E462R; CEEE + Q473K; and CEEE + E462R + Q473K (CEEE + ER + QK) in 10 mM Ba2+ (from left to right). (B) r300 values are shown mean ± SE from −10 to +20 mV for CEEE, CEEE+ E462R; CEEE + E462K; CEEE + Q473K; and CEEE + E462R + Q473K (from left to right). The r300 ratios were strongly voltage-dependent for all constructs. The E462R (29) and E462K (14) point mutations significantly increased the inactivation kinetics of CEEE (26) at p < 10−15 and p < 10−10, respectively. In contrast the inactivation kinetics of CEEE (26) and CEEE + Q473K (12) were not significantly different (p > 0.1). (C) Voltage dependence of inactivation was estimated from isochronal inactivation data points measured after 5 s conditioning pulses. Point mutations at position E462 shifted significantly the voltage dependence of inactivation toward negative potentials from E0.5 = −19 ± 1 mV (13) for CEEE to E0.5 = −35 ± 1 mV (17) for CEEE + E462R (p < 10−4); E0.5 = −30 ± 1 mV (8) for CEEE + E462R + Q473K (p < 10−3); and E0.5 = −28 ± 1 mV (10) for E462K (p < 10−3). In contrast, the Q473K mutation did not significantly alter the voltage dependence of inactivation with E0.5 = −23 ± 1 mV (8). Complete set of fit values are shown in Table 2.