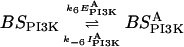TABLE 2.
Reactions and kinetic constants
| Reactions | Rate constants | |
|---|---|---|
|
k0 = 15.6 μM−1 s−1 | |
| k−0 = 0.39 s−1 | ||
|
k1 = 5e-5 (No./μm2)−1 s−1 | |
| k−1 = 0.05 s−1 | ||
|
k2 = 2e-6 (No./μm2)−1 s−1 | |
| k−2 = 2 s−1 | ||
|
k3 = 0.03 (No./μm2)−1 s−1 | |
| k−3 = 30 s−1 | ||
|
k4 = 8e-4 (No./μm2)−1 s−1 | |
| k−4 = 80 s−1 | ||
|
k5 = 1 nM−1 s−1 | |
| k−5 = 8 (No./μm2)−1 s−1 | ||
|
k6 = 1 (No./μm2)−1 s−1 | |
| k−6 = 1 nM−1 s−1 | ||
|
k7 = 1 μM−1 s−1 | |
| k−7 = 1 s−1 | ||
|
k8 = 1 μM−1 s−1 | |
| k−8 = 1 s−1 | ||
|
k9 = 0.01 (No./μm2)−1 s−1 | |
| k−9 = 10 (No./μm2)−1 s−1 |