FIGURE 3.
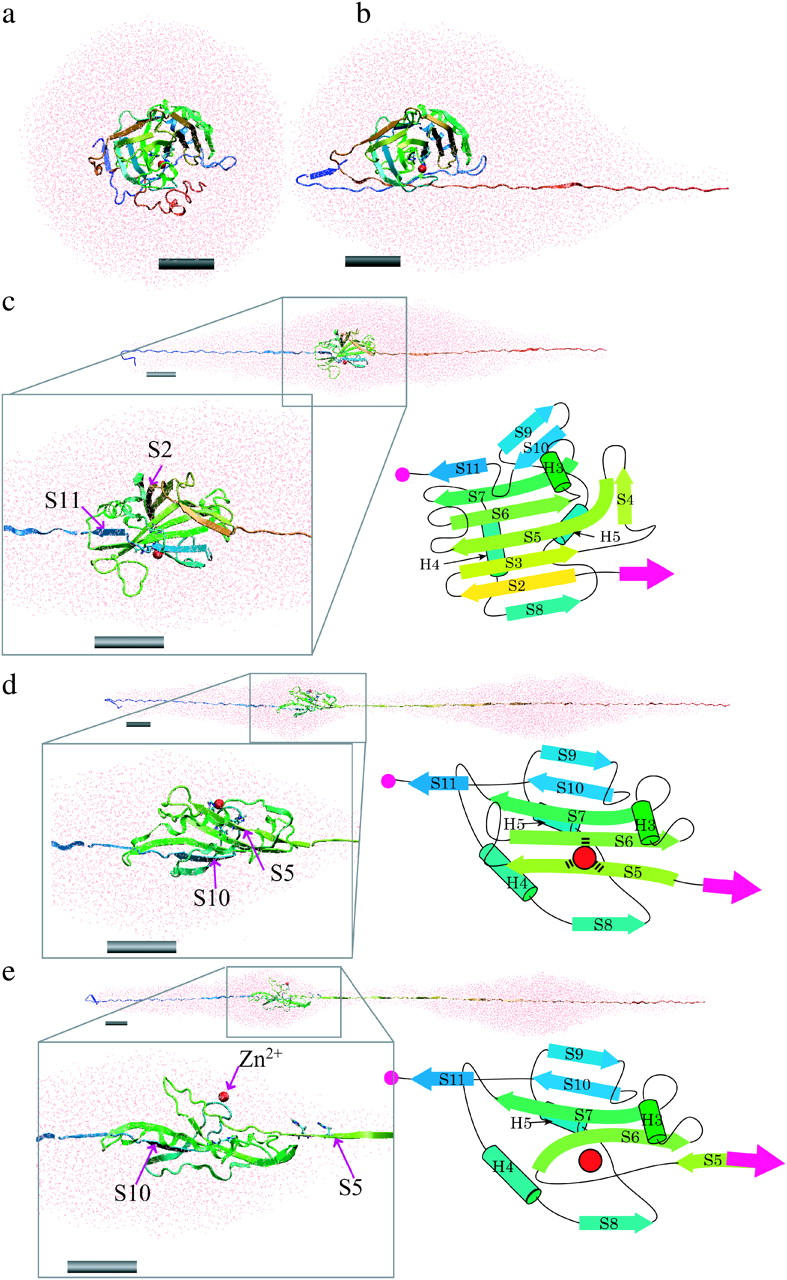
Snapshots showing the structural changes in the protein and surrounding water unfolding as a function of simulation time in the explicit water simulation with a pulling speed of 0.5 Å/ps. (a) The initial structure of the system where the BCAQ253C molecule is soaked in the water droplet. (b) Protein and surrounding water at the 17-nm peak. The unfolding from the C-terminus reached the knot structure, and then the speed of unfolding became slow. (c) At the 40-nm peak. The unfolding of the C-terminal portion pauses at S11, whereas in the N-terminal side, S2 is unfolded. The box in the black line is a magnified view of the remaining β-sheet core containing S2 (yellow arrow) to S11 (blue arrow). A topological diagram of the remaining core is shown in the right of the box. (d) At a 53-nm peak, S5 and S10 pull against each other. The box in the black line is a magnified view of the remaining β-sheet core containing S5 (right green arrow) to S11 (blue arrow). (e) After the 53-nm peak. After the S5 β-strand is pulled apart from the β-sheet, the zinc ion (red sphere) is dissociated from the coordinating histidines. Each gray cylinder represents a scale bar of 2 nm. Figures created by VMD (Humphrey et al., 1996).
