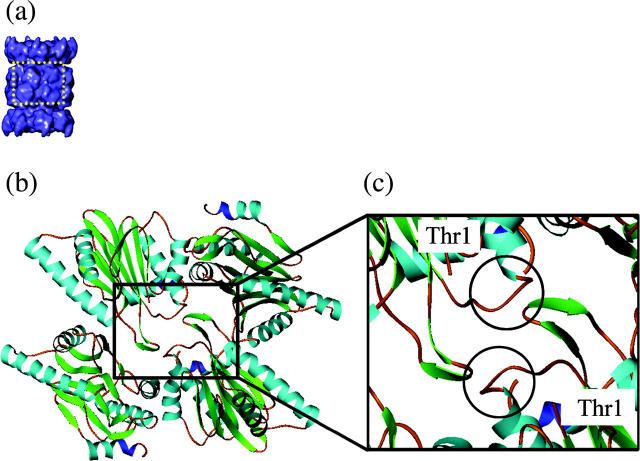
FIGURE 7.
Inter-β-ring subunit contacts and Thr-1 locations in the crystal structure of 20S. (a) The 20S structure from A. fulgidus. (b) Highlighted region of 20S shown as a ribbon diagram of four β-subunits, indicating the β-β-subunit interactions and the location of the catalytic residue, Thr-1. (c) Closeup of the ribbon diagram of four β-subunits with the beak-like domains circled. The beak-like domains (residues 84–96) in one β-ring interact with the β-turn domain (residues 25–30) of the β-subunits in the adjacent β-ring. Due to the arrangement of the rings, the β-subunits interact with each other across a diagonal. There are two regions of interaction between each pair of subunits. All interactions are identical, between the beak-like domain and β-turn domain. The catalytic residue (Thr-1) is shown. Thr-1 is in close proximity to the beak domain.