Figure 3.
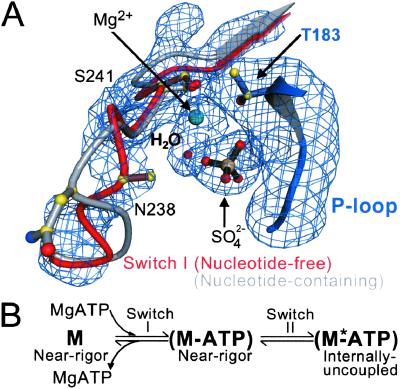
A conformational change in switch I on the binding of nucleotide. (A) Shown is a composite of four difference Fourier (Fo − Fc) electron density omit maps of switch I and the surrounding region of the nucleotide-binding site of the nucleotide-free near-rigor structure. Each map was generated by torsional simulated-annealing in which atoms were omitted either for a segment of switch I (residues N235 to R242), the Mg2+ ion and a water molecule, a sulfate ion, or part of the P loop (residues A180 to T183). The maps are contoured at the 3.5 σ level. Superimposed on the maps is the proposed conformational change of switch I in the transition from the nucleotide-free to the nucleotide-bound (ATP and its analogs, or ADP) states. The P loop of the scallop near-rigor and S1-ADP⋅BeFx structures are superimposed. The “empty” nucleotide-binding site of the near-rigor conformation is shown in color (switch I in red, P loop in blue, Mg2+ ion in green, with a sulfate ion occupying the β-phosphate position). In gray is the same site in the internally uncoupled scallop S1-ADP⋅BeFx structure (nucleotide not shown), which shows the conformation of switch I seen in all nucleotide-containing myosin crystal structures. When nucleotide is present in the binding site, it appears to displace part of switch I, most notably residue N238. The internally uncoupled state is initiated by small changes in the switch II loop (not shown, see ref. 1). (B) The equilibrium equation (in vitro) between the empty and nucleotide-containing near-rigor myosin structures, leading to the internally uncoupled state. Shown also are the steps at which switches I and II change. An asterisk is assigned to the internally uncoupled ATP state (right) to indicate that this conformation may correspond to the second ATP state predicted in biochemical kinetics studies (19). The near-rigor nucleotide-free state (left) is not likely to be part of the in vivo acto-myosin contractile cycle.