Figure 4.
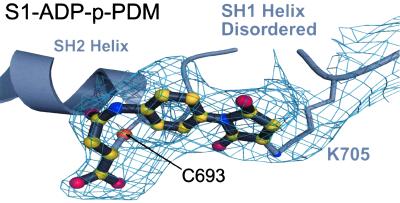
Electron density for the cross-linker. Shown is the simulated annealing Fo − Fc omit map for the electron density of p-PDM together with the cross-linker modeled into the structure of S1-ADP-p-PDM. To generate this map, contoured at the 3.0 σ level, residues C693 and K705 were omitted along with the p-PDM model. The SH1 helix is disordered, including the SH1 sulfhydryl (C703). Biochemical studies with rabbit muscle myosin have indicated that this thiol can be cross-linked to the SH2 sulfhydryl (C693) by p-PDM (15, 24, 25). However, this map reveals that, in scallop S1, p-PDM cross-links the SH2 sulfhydryl to the side chain of K705, instead of SH1. The scallop S1-ATP[γ-S]-p-PDM structure gives the same result.