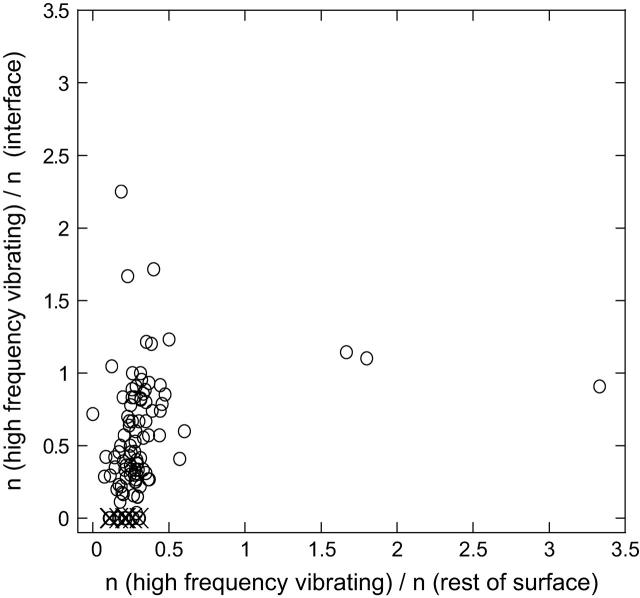
FIGURE 5.
The number of HFV residues contacting with the interface residues versus contacting with the rest of the surface residues for 100 cases. It is normalized by the number of interface residues and the number of residues in the rest of the surface, respectively. The width of the shell and the distance criteria for the interaction are taken as 7 Å; n is used to represent the number of the respective cases. The outliers: 1is7L (hydrolase/protein binding; no conservation neither in interacting nor in nearby residues); 1irxA, B (ligase; a lower threshold value <0.005 of high-frequency peaks is needed to be able to identify HFV residues); 1j46A (oxyreductase; lower threshold required for the fast-mode peaks); 1pmaA, B (protease; multiple interfaces); 1fntC (hydrolase activator; many interfaces); 1dz4A (oxyreductase; two clusters at other binding regions); 1fpuA, B (transferase; a large folding core and a cluster of residues somewhere else on the surface). The outliers are depicted by ×. The number of cases off the diagonal on the space of the noninterface surface residues is 25.