FIGURE 3.
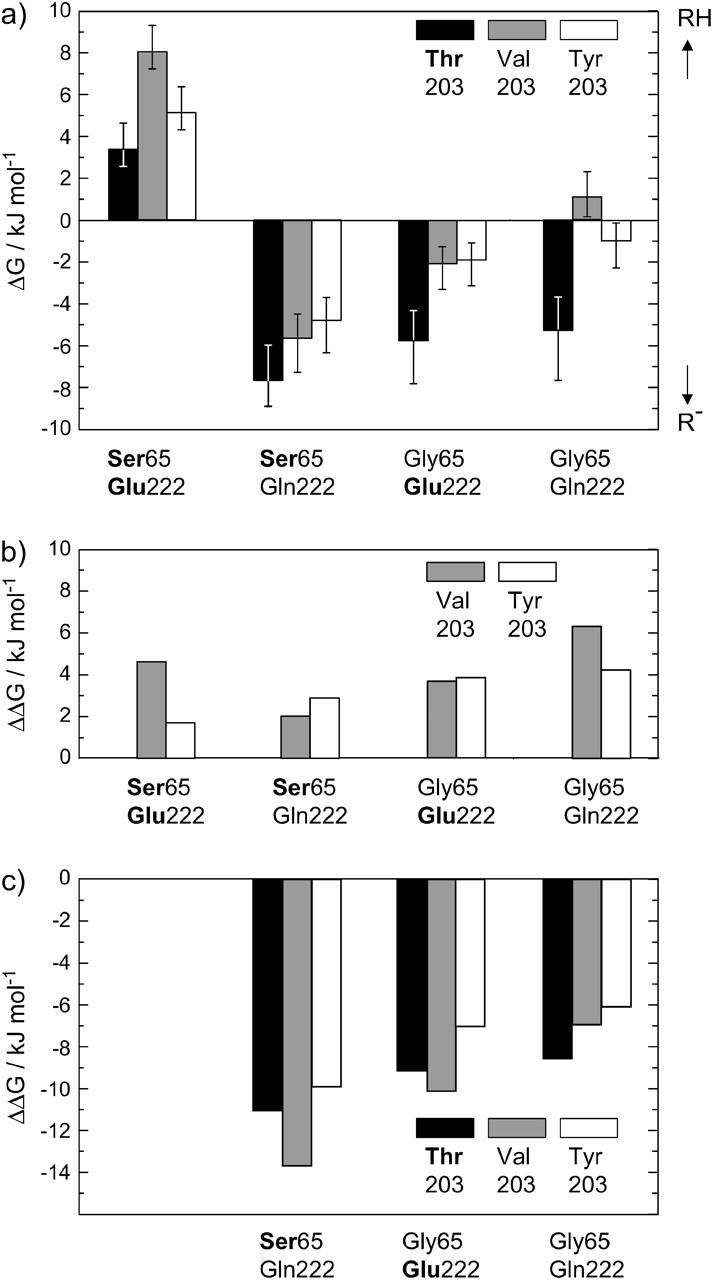
(a) ΔG values for the different chromophore states in the investigated GFP mutants, calculated from the equilibrium constant K. The error bars result from an assumed error of ±40% in the determination of K. An additional error of 50% is included for the mutants with weak RH-absorbance. Bold print denotes the amino acids of wt-GFP. (b) Energy values ΔΔG relative to the T203 variants. The removal of the hydrogen bond between Y66 and the amino acid at position 203 stabilizes the neutral chromophore. (c) Energy values ΔΔG relative to the S65/E222 variants. The perturbation of the hydrogen-bonding network between Y66 and E222 favors the deprotonated chromophore form.
