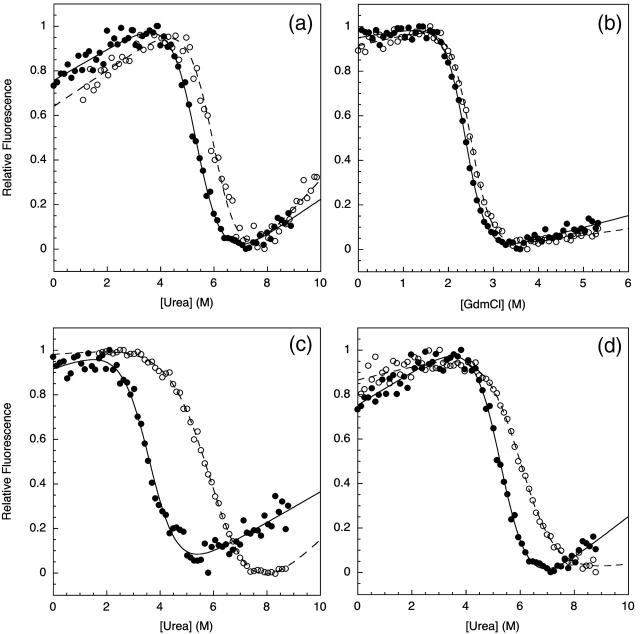
FIGURE 2.
Chemical denaturation measurements comparing the relative stabilities of the TNfn3 monomer (•) and 8-mer construct (○) performed in (a) urea at pH 5, (b) GdmCl at pH 5, and (c) urea at pH 7. Chemical denaturation was monitored by fluorescence at 320 nm at 25°C. (d) The stability of the TNfn3 domain is the same in the TNfn3-I27 8-mer where each TNfn3 domain has an adjacent I27 domain. The apparent change in m-value is due to the contribution of I27 modules in the construct beginning to unfold at the higher denaturant concentrations.