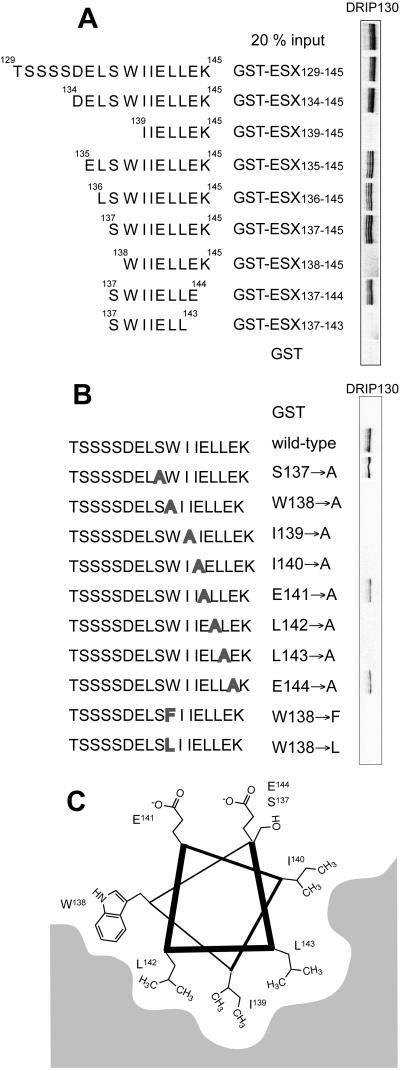
Figure 6.
(A) Identification of the minimal ESX peptide that binds DRIP130 in vitro. It is evident that 35S-labeled DRIP130 binds an 8-aa segment of the ESX activation domain (ESX137–144). (B) Summary of mutational studies. Point mutants of ESX129–145 were tested for their ability to bind DRIP130. Although the Ala substitution of hydrophilic residues had no effect, those of hydrophobic residues in the putative α-helical region abolished the interaction with DRIP130. The indole structure of Trp-138 seems to be important for the interaction because either substitution with Ala, Phe, or Leu abolished the interaction with DRIP130. (C) Projection of the minimal binding peptide, ESX137–144, onto a helical wheel.