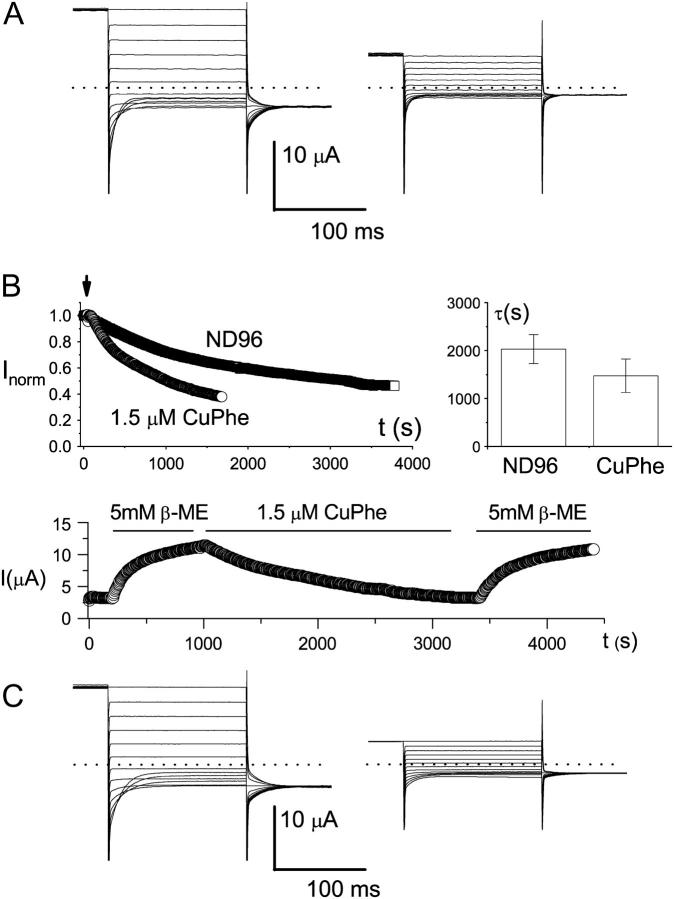
FIGURE 5.
Redox control of the C212S-related mutants. (A) Effects of CuPhe on the C212-C212S heterodimer. Recordings at left were made before, and recordings at right after, treatment with CuPhe (10 μM) for 1 h. Dotted lines represent zero-current level. The number of trace crossovers is 4 on the left and 0 on the right. Five other oocytes showed similar effects. (B) Redox control of the slow-gate open probability of the K165C heterodimer (in C212S background). Shown at upper left is the current reduction of the heterodimer in ND96 with or without 1.5 μM CuPhe. The oocyte was first incubated in β-ME for at least 1 h. The recording was initially made in the same β-ME-containing solution. The arrow indicates the time when the solution was replaced with pure ND96 solution, or ND96 solution with 1.5 μM CuPhe. The current was measured at +40 mV using protocol 1, and the amplitudes were normalized to the current amplitudes just before the solution exchange. The graph in the upper right corner gives a comparison of the current reduction rate in the absence or presence of 1.5 μM CuPhe. Time constants were derived from a single-exponential fit of the recordings like those shown on the left. The bottom panel shows how inhibition of the K165C heterodimer, both by spontaneous oxidation and by the applied CuPhe, could be reversed by the reducing reagent β-ME. The dotted line represents zero-current level. The oocyte was initially recorded in ND96, and the redox reagents were applied as shown by the horizontal lines. (C) Redox control of K165-K165E heterodimer (in C212S background). Recordings at left were made before, and recordings at right after, 1 h of CuPhe (10 μM) treatment. Three other oocytes showed similar effects. In addition, five oocytes expressing K165-K165H heterodimer (also in C212S background) also showed similar effects of CuPhe.