Abstract
We have measured the rates of insertion into, desorption from, and spontaneous interlayer translocation (flip-flop) of the fluorescent lysophospholipid derivative NBD-lyso-1-myristoylphosphatidylethanolamine in ld and lo phase lipid bilayer membranes. The lipid bilayers, studied as LUV, were prepared from pure 1-palmitoyl-2-oleoylphosphatidylcholine, in the ld phase; and from two Chol-containing binary lipid mixtures, 1-palmitoyl-2-oleoylphosphatidylcholine and Chol (molar ratio of 1:1) and SpM and Chol (molar ratio of 6:4), both in the lo phase. Insertion, desorption, and translocation rate constants and equilibrium constants for association of the amphiphile monomer with the lipid bilayers were measured between 15°C and 35°C, and the standard free energies, enthalpies, and entropies, as well as the activation energies for these processes were derived from these data. The equilibrium partition coefficients for partitioning of the amphiphile between the aqueous phase and the different membrane phases were also derived, and an estimation was made of hypothetical partition coefficients and the respective energetic parameters for partitioning between the different lipid phases if these were to coexist in the same membrane. We show that, contrary to general belief, the association of NBD-lysoMPE with lipid bilayers is not a diffusion-controlled process, the rate-limiting step in insertion being the formation of a free area in the membrane surface of an adequate size for insertion to occur.
INTRODUCTION
The kinetics and thermodynamics of the association of amphiphilic molecules with lipid bilayers, biological membranes, and other organized polar lipid surfaces can provide information concerning the physical properties of those systems. They may also serve as the basis for the understanding of physiological processes such as lipid exchange between cellular membranes, transmembrane translocation of lipids and other amphiphilic molecules across membranes, and the exchange of lipids and other amphiphiles between lipid particles and membranes. In particular, the energetics of lipid amphiphile interaction with lipid bilayers has an important predictive value. It is with this reasoning that we have been studying the interaction of fluorescent lipid derivatives and other amphiphilic molecules with lipid bilayers in different physical states over the past few years (Abreu et al., 2003, 2004; Estronca et al., 2002, 2005; Mesquita et al., 2000; Pokorny et al., 2000, 2001; Vaz and Melo, 2001; Vaz et al., 2004). The emphasis in this work is threefold: 1), to understand the rules that govern the partitioning of amphiphiles between membranes of different phases or between different phases coexisting in the same membrane; 2), to study the rates of all processes involved in the association of lipid-like molecules in lipid bilayer membranes in different phases and in particular in ld and lo phases; and 3), from kinetic and equilibrium association studies, to arrive at the thermodynamics of these processes including the energetics of passage through the transition states.
To obtain physical data that can be extrapolated to understand similar interaction of membranes with amphiphilic substances that are not amenable to direct study, it is useful to obtain data on the interaction of homologous series of amphiphiles with membranes. In the case of lipid-derived amphiphilic molecules, at least three homologous series can be studied: 1), phospholipid derivatives with one, two, or more hydrophobic acyl chains of the same size and degree of saturation, being similar in all other respects; 2), phospholipid derivatives that are similar in all respects except with varying acyl chain lengths and degree of saturation; and 3), phospholipid derivatives with the same apolar structure and different headgroups. In recent work (Abreu et al., 2004), we described the association of a fluorescent diacylglycerophospholipid derivative, NBD-DMPE with lipid bilayers in disordered Lα phases (the so-called ld phases) and in ordered Lα phases (the so-called lo phases). In this work, we performed an identical study with the single-chain homolog of this fluorescent phospholipid, namely, NBD-lysoMPE. Similar to the previous work, the entire study was done as a function of temperature between 15°C and 35°C, which permitted us to derive the thermodynamic variables of all equilibrium and activation processes. Also, as in the previous work, three types of lipid bilayer membranes were studied: lipid bilayers in the ld phase prepared from pure POPC, and two Chol-rich lipid bilayers in the lo phase prepared from an equimolar mixture of POPC and Chol and from a 6:4 molar ratio of SpM and Chol.
The exchange of lipids and lipid derivatives between lipid bilayer vesicles has been studied for at least the last 30 years. Most of this work has examined the exchange of amphiphilic molecules between a donor and an acceptor population. The measured efflux rates were shown in almost all cases, not surprisingly, to be first order processes. In all of this work, the insertion rate has been assumed to be much faster than the efflux rate. Having measured both the insertion and desorption rate constants for amphiphile association with membranes, our results show that this assumption is valid. In several cases reported in the literature, the insertion rate constant was assumed, although never demonstrated, to be a diffusion-controlled process. This assumption probably derives from the demonstration in the 1970s (Aniansson et al., 1976) that the association rate of anionic amphiphile monomers to form micelles was “very close to being diffusion controlled”. In recent work (Abreu et al., 2004), we have shown that the insertion of NBD-DMPE into lipid bilayers is very clearly not diffusion controlled. In this work we show that the insertion of NBD-lysoMPE into lipid bilayers, although very rapid, is also not a diffusion-controlled process. We shall present a model which explains this result.
Excluding our own work in this area (Abreu et al., 2003, 2004; Estronca et al., 2005; Pokorny et al., 2000, 2001), there are practically no reports in the literature (see Nichols, 1985 for a notable exception) in which the complete kinetics and thermodynamics of amphiphile association with lipid bilayers has been described without making any assumptions. The detail of these results allows us to propose a mechanism for the association of amphiphiles with membranes.
MATERIALS AND METHODS
NBD-DMPE (purity >99%) and POPC were from Avanti Polar Lipids (Alabaster, AL); egg yolk SpM and porcine phospholipase A2 were from Sigma-Aldrich Química S.A. (Sintra, Portugal); and Chol was from Serva/Boehringer Ingelheim (Heidelberg, Germany). All reagents were of the highest commercially available purity. Solvents of analytical reagent grade were from Merck Portuguesa (Lisbon, Portugal).
NBD-lysoMPE was prepared from NBD-DMPE by treatment with phospholipase A2 as described by Eibl et al. (1983). The product (NBD-lysoMPE) was separated from the reaction mixture using preparative thin layer chromatography on silica gel 60 plates (Merck Portuguesa) using a mixture of chloroform, methanol, and water (65:25:4, v/v/v) as the eluant. NBD-lysoMPE was eluted off the thin layer and stored at −20°C in the dry state since this product was found to be unstable (one of the reaction products being N-NBD-ethanolamine), particularly in aqueous solution. The degradation of NBD-lysoMPE in aqueous solution was followed qualitatively by examining for the formation of NBD-ethanolamine. At 35°C, no NBD-ethanolamine was formed during a period of 8 h, trace amounts of this product being detectable after 24 h at this temperature. At lower temperatures this degradation was considerably slower, no NBD-ethanolamine being detectable over a period of 3 days at 25°C. In view of this instability, aqueous solutions of NBD-lysoMPE were always freshly prepared from the dry stock of this product. All aqueous solutions of NBD-lysoMPE were prepared by addition of 10 mM sodium phosphate, 0.15 M sodium chloride, 0.02% sodium azide, pH 7.4 buffer to a dried film containing a previously determined amount of NBD-lysoMPE.
Phospholipid concentrations were determined using a modified version of the Bartlett phosphate assay (Bartlett, 1959), and Chol concentrations were determined by the Lieberman-Burchard method as described by Taylor et al. (1978). NBD-lysoMPE concentration was spectrophotometrically determined in methanol solutions assuming a molar extinction coefficient of 21,000 M−1cm−1 at 463 nm. Absorption spectra were recorded on a Unicam UV530 UV/Vis spectrophotometer (Cambridge, UK), and steady-state fluorescence measurements were performed on a Cary Eclipse Fluorescence Spectrophotometer (Victoria, Australia) equipped with a thermostatized cell holder.
Aqueous suspensions of lipids were prepared by evaporating a solution of the desired lipid or lipid mixture premixed in an azeotropic mixture of chloroform and methanol by blowing dry nitrogen over the heated (blowing hot air over the external surface of the tube) solution and then leaving the residue in a vacuum dessicator for at least 8 h at 23°C. The solvent-free residue, heated in a water bath at 60°C, was then hydrated with an aqueous solution of 0.15 M sodium chloride containing 0.02% sodium azide, which had been previously heated to the same temperature, and the mixture was left to hydrate for ∼10 min at 60°C. The amount of hydrating medium added was calculated to result in a final lipid concentration of ∼4 × 10−3 M. The hydrated lipid was vigorously vortexed at room temperature to produce a suspension of multilamellar vesicles that was then extruded, using a minimum of 10 passes, through two stacked polycarbonate filters (Nucleopore, Whatman, Springfield Hill, UK) with a pore diameter of 0.1 μm (Hope et al., 1985). During extrusion, the water-jacketed extruder (Lipex Biomembranes, British Columbia, Canada) was maintained at a temperature of 65°C (for membranes prepared from mixtures of SpM and Chol) or 23°C (for other membranes). The LUV suspensions obtained after extrusion were diluted in buffer to obtain the desired lipid concentration for fluorimetric experiments. LUV concentrations were calculated from the total lipid concentration assuming a LUV diameter of 0.1 μm and the mean area per lipid molecule reported from monolayer experiments at a lateral surface pressure of 30 mN m−1 (Smaby et al., 1997 and work cited therein).
In preliminary experiments it was determined that NBD-lysoMPE did not form aggregates or micelles at least up to a concentration of 10−6 M, which is in agreement with the value of 4 × 10−6 M for the CAC of this amphiphile reported by Shoemaker and Nichols (1990).
Equilibrium titration of a 2 × 10−7 M solution of NBD-lysoMPE in 10 mM sodium phosphate, 0.15 M sodium chloride, 0.02% sodium azide, pH 7.4 buffer was done by adding LUV to the desired concentration. The mixtures were allowed to equilibrate for at least 24 h at the desired temperature before fluorescence spectra were measured. The equilibrium association constant, KL, was obtained by fitting the experimental points to the appropriate theoretical expression using a least-squares minimization procedure. Values of KL were obtained at least in triplicate ab initio experiments at each of the experimental temperatures.
Stopped-flow measurements were performed on a thermostated stopped-flow fluorimeter (Hi-Tech model SF-61, Salisbury, UK) by mixing equal volumes of thermally preequilibrated solutions of 2 × 10−7 M NBD-lysoMPE in buffer with a suspension of LUV (lipid concentration of 1 × 10−4 M) in the same buffer. The excitation beam was monochromated at 450 nm, and emission was observed through a 3 mm thick OG530 cutoff filter (Schott, Mainz, Germany). Data were obtained at a time resolution of 1 ms over a total period of between 0.3 and 1 s, and occasional curves were obtained at lower resolution over a period of up to 5 s to assure that equilibrium had been achieved. The experimental curves of change in fluorescence intensity in time were analyzed by least-squares fitting of a theoretical description of the process (see below) using Solver from Microsoft Excel 2003.
The rate of spontaneous translocation of NBD-lysoMPE between the monolayers of a LUV was measured by studying the time course of reduction of the NBD groups located in the outer monolayer of LUV using sodium dithionite (McIntyre and Sleight, 1991). Briefly, a suspension of LUV of the desired composition in 0.01 M HEPES, pH 7.4, was equilibrated with NBD-lysoMPE at 25°C for 48 h. At the end of this period the mixture was equilibrated for 1 h at the desired temperature, and 20 μL of a freshly prepared 1 M sodium dithionite solution in 1 M Tris-HCl, pH 10, was added to 1 mL of the suspension. The NBD groups in the aqueous phase and in the outer monolayer of the LUV were rapidly (within a few minutes) reduced, followed by a slow reduction (typically over hours) of the probe that was initially in the inner monolayer of the LUV and spontaneously translocated to the outer monolayer. The process was followed continuously in time to yield the translocation rate constant either from an analysis of the entire bleaching curve or from the initial velocity.
RESULTS
Equilibrium association of NBD-lysoMPE with LUV
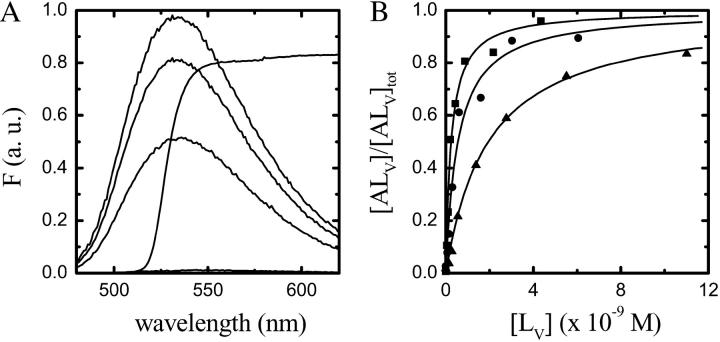
The relative fluorescence quantum yield of NBD-lysoMPE increases ∼100-fold (for the case of POPC bilayers) upon transferring this amphiphile from an aqueous solution (in which it exists as a monomer) to a membrane phase. This result, shown in Fig. 1 A, was used to assess the equilibrium constant, KL, for the association of NBD-lysoMPE with LUV membranes (Fig. 1 B) in the ld phase prepared from pure POPC, and in the lo phase prepared from a binary mixture of POPC and Chol (1:1 molar ratio) and a binary mixture of SpM and Chol (6:4 molar ratio). The association was assumed to proceed according to the equation
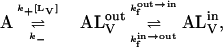 |
(1) |
where A is the amphiphile monomer in aqueous solution, 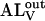 and
and 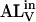 are NBD-lysoMPE associated with the outer and inner monolayers, respectively, LV is the LUV, k+[LV] and k− are the association and dissociation rate constants, and
are NBD-lysoMPE associated with the outer and inner monolayers, respectively, LV is the LUV, k+[LV] and k− are the association and dissociation rate constants, and 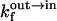 and
and 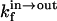 are the rate constants for the spontaneous translocation of the amphiphile between the two monolayers of the LUV in the indicated directions. The association rate constant is a pseudo-first order rate constant that depends upon the concentration of LUV in the reaction system, and the LUV are assumed not to be consumed in the reaction since at low values of
are the rate constants for the spontaneous translocation of the amphiphile between the two monolayers of the LUV in the indicated directions. The association rate constant is a pseudo-first order rate constant that depends upon the concentration of LUV in the reaction system, and the LUV are assumed not to be consumed in the reaction since at low values of 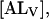 the LUV containing A are just as capable of reaction with A as LUV without A. The total concentration of NBD-lysoMPE associated with the LUV membranes at equilibrium is
the LUV containing A are just as capable of reaction with A as LUV without A. The total concentration of NBD-lysoMPE associated with the LUV membranes at equilibrium is 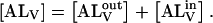 If the inner and outer monolayers of the LUV have about equal areas, which is approximately the case for the LUV we use,
If the inner and outer monolayers of the LUV have about equal areas, which is approximately the case for the LUV we use, 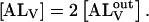 The equilibrium association constant is given by
The equilibrium association constant is given by
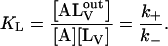 |
(1a) |
A, originally present in the aqueous phase outside the LUV, reaches the inner monolayer by passive translocation across the lipid bilayers of the LUV and will equilibrate with the internal aqueous phase of the LUV as well, but since the volume of this compartment is very small relative to the total volume of the aqueous phase, it may be ignored. The translocation equilibrium constant, KF, is given by
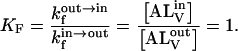 |
(1b) |
From the above, it can be shown that at equilibrium the total amount of NBD-lysoMPE associated with the lipid bilayers, 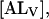 is given by
is given by
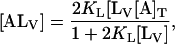 |
(1c) |
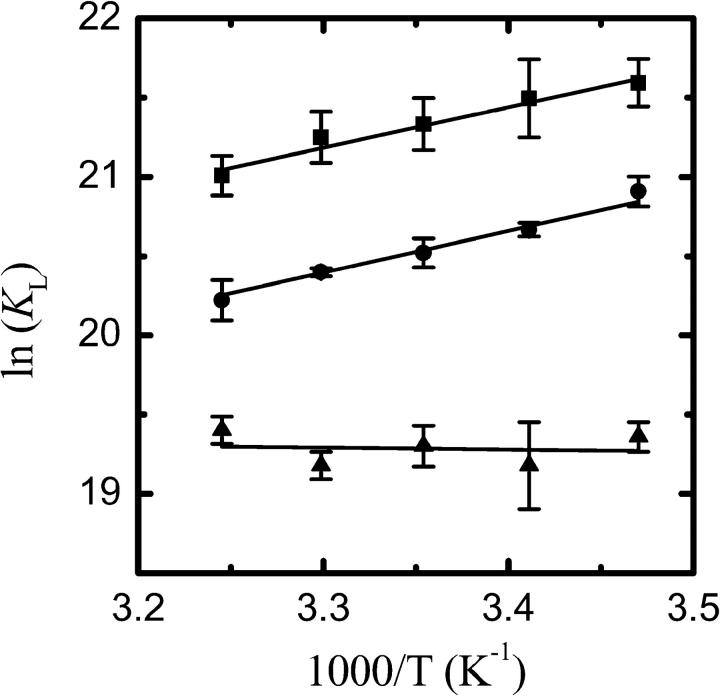
where 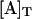 is the total concentration of NBD-lysoMPE in the system. KL was determined as a function of temperature between 15°C and 35°C. The results are shown as van't Hoff plots in Fig. 2, and the result at 35°C is listed in Table 1.
is the total concentration of NBD-lysoMPE in the system. KL was determined as a function of temperature between 15°C and 35°C. The results are shown as van't Hoff plots in Fig. 2, and the result at 35°C is listed in Table 1.
FIGURE 1.
(A) Fluorescence emission spectra of NBD-lysoMPE (2 × 10−7 M) in aqueous solution (lowest trace) and associated with POPC LUV (at concentrations of 2.2 × 10−10 M, 8.7 × 10−10 M, and 4.4 × 10−9 M, respectively, from bottom to top). The spectrum of the emission cutoff filter in the stopped-flow experiments is shown as broken lines. (B) Titration curve of a 2 × 10−7 M aqueous solution of NBD-lysoMPE with LUV suspensions at 25°C. The curves shown are for LUV prepared from POPC (▪), POPC-Chol (1:1; •), and SpM-Chol (6:4; ▴). The lines are theoretical fits to the experimental points.
FIGURE 2.
Van't Hoff plots for the association of NBD-lysoMPE with LUV prepared from pure POPC (▪), POPC-Chol (1:1; •), and SpM-Chol (6:4; ▴).
TABLE 1.
Kinetic and thermodynamic constants for association (insertion and desorption) and translocation of NBD-lysoMPE within lipid bilayer membranes at 308 K
| POPC | POPC-Chol (1:1) | SpM-Chol (6:4) | |
|---|---|---|---|
| k+ (M−1 s−1) | (3.4 ± 0.3) × 1010 | (1.7 ± 0.1) × 1010 | (1.7 ± 0.1) × 109 |
| k− (s−1) | 25 ± 2 | 27 ± 2 | 7.2 ± 0.3 |
| kf (s−1) | 6.4 × 10−4 | 2.7 × 10−4 | 4.2 × 10−5 |
| KL (M−1) | 1.3 × 109 | 6.1 × 108 | 2.7 × 108 |
| KP(L/W) | 1.6 × 104 | 7.7 × 103 | 3.4 × 103 |
| ΔGo (kJ mol−1) | −54 | −52 | −49 |
| ΔHo (kJ mol−1) | −21 | −22 | 1 |
| TΔSo (kJ mol−1) | 33 | 30 | 50 |
| Eact (desorb) (kJ mol−1) | 47 | 38 | 69 |
| Eact (insert) (kJ mol−1) | 26 | 16 | 70 |
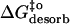 (kJ mol−1) (kJ mol−1) |
67 | 67 | 71 |
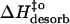 (kJ mol−1) (kJ mol−1) |
44 | 36 | 67 |
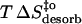 (kJ mol−1) (kJ mol−1) |
−23 | −32 | −4 |
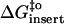 (kJ mol−1) (kJ mol−1) |
13 | 15 | 21 |
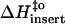 (kJ mol−1) (kJ mol−1) |
23 | 14 | 68 |
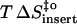 (kJ mol−1) (kJ mol−1) |
10 | −1 | 47 |
| Eact (transloc) (kJ mol−1) | 104 | 112 | 48 |
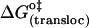 (kJ mol−1) (kJ mol−1) |
95 | 97 | 101 |
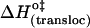 (kJ mol−1) (kJ mol−1) |
102 | 106 | 45 |
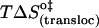 (kJ mol−1) (kJ mol−1) |
7 | 9 | −57 |
Kinetics of association of NBD-lysoMPE with LUV
The time dependence of NBD-lysoMPE association with LUV membranes was studied by stopped-flow mixing fluorimetry as a function of temperature between 15°C and 35°C. Fig. 3 shows typical association kinetic curves for the three types of LUV studied. The traces are clearly monoexponential as would be expected if the association proceeded according to Eq. 1 and the translocation rate were much slower than the rate of association with the membrane so that the translocation rate constants would effectively be negligible. The rate of change of concentration of each of the NBD-lysoMPE species in Eq. 1 may be given by the following differential equations
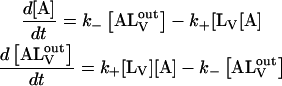 |
(2) |
that can be integrated to give the time dependence of each of the NBD-lysoMPE species. In the experiments described here, the changes in concentration of the relevant species are followed by measuring their fluorescence intensity. Since the fluorescence of NBD-lysoMPE in the aqueous phase is negligible compared to that of the amphiphile in the membrane (see Fig. 1 A), the signal emanating from the former species may be ignored. Also, if it can be shown that the first equilibrium in Eq. 1 is achieved at a rate that is very much faster than the rate of the translocation process (second equilibrium in Eq. 1), the second equilibrium can be ignored and essentially we only follow the temporal evolution of 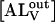 in the stopped-flow experiment. This condition will be shown below to hold in these experiments. The temporal evolution of
in the stopped-flow experiment. This condition will be shown below to hold in these experiments. The temporal evolution of 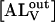 is given by
is given by
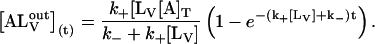 |
(3) |
FIGURE 3.
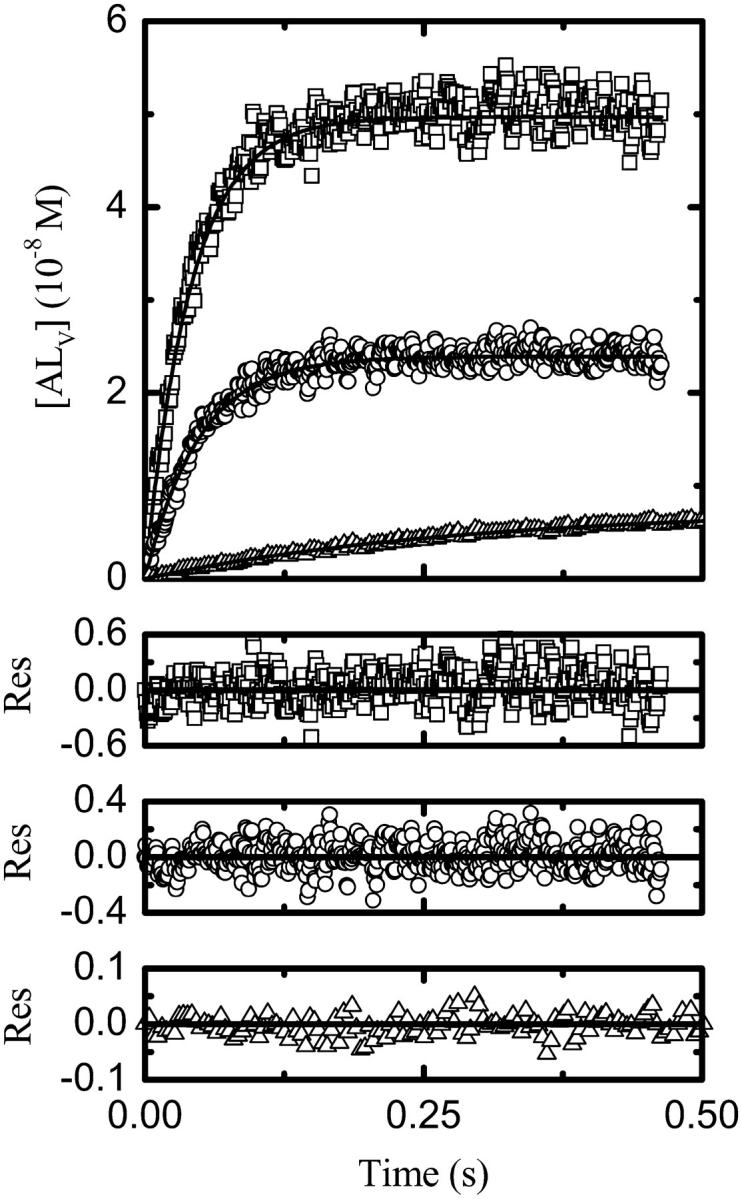
Experimental kinetic traces at 25°C for the association of NBD-lysoMPE with LUV prepared from POPC (□), POPC-Chol (1:1; ○), and SpM-Chol (6:4; Δ) with their respective theoretical fits.
In the stopped-flow experiments, we measure the fluorescence intensity of NBD-lysoMPE that is inserted into the outer monolayer of the LUV. The fluorescence at any given time during the reaction, F(t), is given by
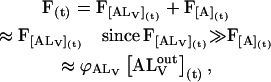 |
(4) |
where Fi(t) is the fluorescence intensity due to the ith species at time t. The values of  were obtained from calibration curves between 15°C and 35°C: NBD-lysoMPE was premixed in chloroform-methanol solution with the lipid (POPC and POPC/Chol), and LUV were prepared from the solvent-free residue of the mixture as described in Materials and Methods. The ratio of probe to lipid was adjusted by mixing a premixed fluorescent LUV preparation with the corresponding probe-free LUV and incubating for 24 h at 25°C. The final total lipid concentration in the calibration curves was 1 mM, which corresponds to at least 90% of the probe being in the lipid bilayers. This procedure was not viable in the case of SpM/Chol due to the instability of NBD-lysoMPE at the high temperatures required in the extrusion of these bilayers. In this case NBD-lysoMPE was added to preformed SpM/Chol LUV and incubated for 48 h at 25°C. Parallel treatment of POPC and POPC/Chol LUV showed the two procedures to be equivalent.
were obtained from calibration curves between 15°C and 35°C: NBD-lysoMPE was premixed in chloroform-methanol solution with the lipid (POPC and POPC/Chol), and LUV were prepared from the solvent-free residue of the mixture as described in Materials and Methods. The ratio of probe to lipid was adjusted by mixing a premixed fluorescent LUV preparation with the corresponding probe-free LUV and incubating for 24 h at 25°C. The final total lipid concentration in the calibration curves was 1 mM, which corresponds to at least 90% of the probe being in the lipid bilayers. This procedure was not viable in the case of SpM/Chol due to the instability of NBD-lysoMPE at the high temperatures required in the extrusion of these bilayers. In this case NBD-lysoMPE was added to preformed SpM/Chol LUV and incubated for 48 h at 25°C. Parallel treatment of POPC and POPC/Chol LUV showed the two procedures to be equivalent.
The experimental kinetic curves were fitted with theoretical curves described by Eq. 4 using a least-squares fitting procedure, and the values of k+ and k− were extracted from the best fits. We note that the only adjusting parameter in the kinetic curves was k+ since KL was independently known.
Fig. 4 shows Arrhenius plots for k+ and k− for association of NBD-lysoMPE with each of the three bilayer types examined. The results are summarized for 35°C in Table 1.
FIGURE 4.
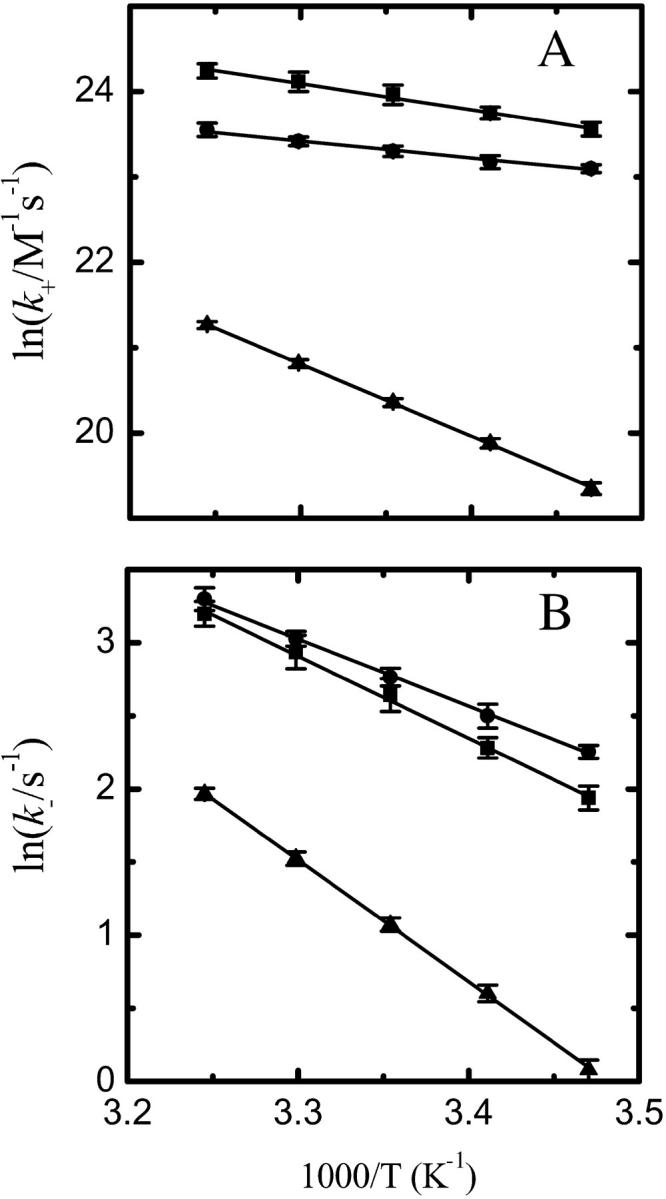
Arrhenius plots for NBD-lysoMPE association with LUV bilayers. Data are shown for (A) the insertion process (k+) and (B) the desorption process (k−), with LUV prepared from pure POPC (▪), POPC/Chol (1:1) (•), and SpM/Chol (6:4) (▴).
Spontaneous transbilayer translocation (flip-flop) of NBD-lysoMPE
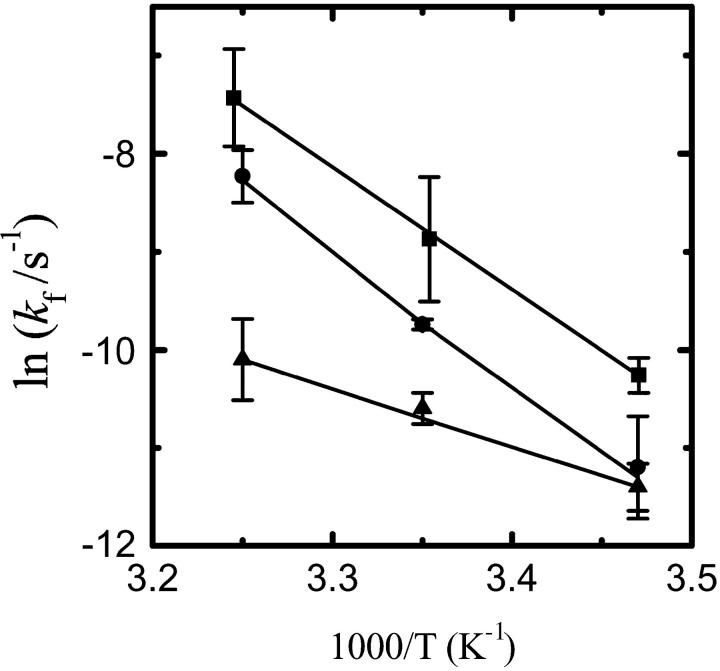
In this series of experiments we used an established technique (McIntyre and Sleight, 1991) to study the spontaneous transbilayer translocation of NBD-lysoMPE in the lipid bilayers examined. In studying the translocation of NBD-DMPE (Abreu et al., 2004), the fluorescent amphiphile was premixed in organic solvent with the lipids that constituted the bilayers, and LUV were prepared by extrusion of the multilamellar liposomes formed upon hydration of the lipid. This procedure could not be used in the case of NBD-lysoMPE due to its instability in aqueous solutions at high temperatures. This was especially the case with bilayers prepared from SpM/Chol (6:4) mixtures because the extrusion of these bilayers is normally done at 65°C and takes a few hours to complete. An alternative procedure was, therefore, used: The LUV were incubated with NBD-lysoMPE over a 48 h period of time at 23°C. This time was long enough to ensure that at least part of the fluorescent amphiphile had translocated from the outer leaflet (into which it is rapidly incorporated, see above) to the inner leaflet of the LUV. These reaction mixtures were then brought up to the desired temperature, and dithionite was added to reduce the NBD chromophore to a nonfluorescent species. All of the NBD-lysoMPE in the aqueous phase and outer leaflet of the LUV was bleached in a few minutes, and this was followed by a slow bleach of the remaining NBD-lysoMPE in which the rate-limiting step is the slow translocation of the amphiphile from the inner to the outer leaflet. The results are shown in Fig. 5 as Arrhenius plots. As reported elsewhere (Abreu et al., 2004), we used the initial slope of the slow bleaching phase to obtain kf. The values of kf at 35°C and the activation energy of the translocation process are also listed in Table 1.
FIGURE 5.
Arrhenius plots for the interbilayer translocation of NBD-lysoMPE in LUV bilayers. Results are shown for LUV prepared from pure POPC (▪), POPC/Chol (1:1) (•), and SpM/Chol (6:4) (▴).
DISCUSSION
This report deals with the kinetics and thermodynamics of the association of NBD-lysoMPE with lipid bilayers in the ld phase (prepared from pure POPC) and in the lo phase (prepared from binary mixtures of POPC and Chol (at a molar ratio of 1:1) and from SpM and Chol (at a molar ratio of 6:4)). It is a companion to a previous report from this laboratory (Abreu et al., 2004) on the association of a two-chain homolog, NBD-DMPE, of the amphiphile used in this work. This study was made considerably simpler than the previous one due to the fact that NBD-lysoMPE has a CAC of ∼4 × 10−6 M (Heerklotz and Epand, 2001; Høyrup et al., 2001; Shoemaker and Nichols, 1990). Thus, it exists exclusively as a monomer in aqueous solution at the concentrations (∼10−7 M) that were used here, whereas NBD-DMPE has a very low CAC and its study required a complicated strategy for its monomerization in the aqueous solution (Abreu et al., 2003). In what follows, these results will be discussed with reference to the results on the association of NBD-DMPE with the same lipid bilayers, and an attempt will be made to understand them both in terms of the structure of these homologs and the physical properties of the lipid bilayers that condition their interaction with the membranes.
There is a vast literature on some aspects of the kinetics of lipid-derived amphiphile association with lipid bilayers and biological membranes. Generally these reports are limited to the study of transfer of amphiphiles from a donor to an acceptor population. Most of that work measures only the first order rate-limiting step, namely, desorption from the donor. Insertion of the amphiphiles into the acceptor population is assumed to be much faster than desorption and, in some cases, it is even assumed to be diffusion limited. Besides this work and our own earlier report on the association of NBD-DMPE with lipid bilayers (Abreu et al., 2004), we are aware of only one other report in the literature (Nichols, 1985) in which all the kinetic constants of lipid-derived amphiphile association with lipid bilayer membranes were experimentally measured.
The most striking difference in the kinetics of association of NBD-lysoMPE as compared with NBD-DMPE is in the rate constants of the insertion and desorption processes (k+ and k−). The single-chain amphiphile inserts into the bilayers, and particularly into the ld phase POPC bilayers, with rate constants that are very close to the diffusion-controlled limit (∼2 × 1011 M−1 s−1). However, it must be emphasized that the insertion rate constants for NBD-lysoMPE are not diffusion controlled. This becomes particularly evident from the fact that k+ is not the same for association with the three types of LUV examined (see Table 1). We recall that k+ for NBD-DMPE association with the same bilayers was almost uniformly four orders of magnitude smaller than expected for a diffusion-controlled process. The two values can be understood in terms of the mechanism of insertion of the amphiphiles into the bilayers. An encounter complex between the two reacting species (amphiphile monomer and lipid vesicle) is formed at a diffusion-limited rate, but insertion of the amphiphile into the bilayer is dependent upon the bilayer presenting a hole (free area on the surface) of adequate size for insertion of the surface-associated amphiphile to occur and also upon the orientation of the amphiphile relative to this free area. The free area results from random density fluctuations in the bilayer liquid and can be described by the free volume (area) theory for liquids (Cohen and Turnbull, 1959; Eyring and Ree, 1961) as adapted for liquid-phase bilayer membranes (Galla et al., 1979; Vaz et al., 1985). Thus, the insertion rate constant is determined by the probability of encountering a free area in the membrane liquid surface, which has a dimension approximately equal to the cross sectional area of a single alkyl chain (NBD-lysoMPE) or two alkyl chains (NBD-DMPE). This probability is a physical property of the bilayer liquid phase. We have discussed this in greater detail elsewhere (Vaz et al., 2004).
The rate constants for desorption of NBD-lysoMPE are also ∼5−8 × 105-fold larger than they are for NBD-DMPE. This large difference probably has its origin in the loose fit and consequently weaker intermolecular interactions of the single-chain amphiphile in the lipid bilayers, which seems to be borne out by the thermodynamics of the processes involved (see below).
The only other work in the literature which reports both rate constants (k+ and k−) and the equilibrium constant (KL) in the association of lipid-derived amphiphiles with membranes is that of Nichols (1985). This author studied the association of alkyl chain-modified phosphatidylcholines (NBD-PCs) with dioleoylphosphatidylcholine vesicles prepared by a solvent-injection protocol. The reported value of k+, assuming a vesicle diameter of 50 nm, was 3.4 × 1010 M−1s−1, which is identical to the value we obtain for the rate constant of NBD-lysoMPE insertion into POPC bilayers. Nichols' conclusion that this “rate constant is of the magnitude expected for diffusion-limited rate processes in water” is qualitatively correct but quantitatively flawed—it is ∼5 times slower than expected for a diffusion-limited process. This fact is borne out in the observation that the enthalpy of activation observed by this author is “significantly larger than expected for a diffusion-limited process in bulk water”. Nichols' value for k− is ∼4 × 10−2s−1, which lies somewhere between our results for NBD-lysoMPE (Table 1) and those for NBD-DMPE (Abreu et al., 2004). In making this comparison we note that the NBD group attached to a primary amine is very hydrophilic. NBD-ethanolamine, for example, has an octanol/water partition coefficient of ∼2 × 10−3 (M. J. Moreno, unpublished results). This results in its equilibrium position in a lipid bilayer, when incorporated as NBD-PC, being ill defined but predominantly at the bilayer interface (Huster et al., 2001) and in unknown effects upon the kinetics of association of NBD-PC with lipid bilayers. On the other hand, the kinetics of association (in particular, of insertion) of the headgroup-modified NBD-PE derivatives (our case) with membranes may be expected to be less affected by the perturbations induced by the reporter group, which has been shown to reside approximately at the location of the choline moiety of a phosphatidylcholine molecule in the bilayer (Abrams and London, 1993; Chattopadhyay and London, 1987). The dipole moment of the probe also agrees in orientation with the dipolar potential of the membrane surface (Estronca et al., 2002), avoiding significant conflict with the orientation of the hydration layer at the membrane surface. Thus, for example, KL for association of lyso-1-myristoylphosphatidylcholine measured by isothermal titration calorimetry (Høyrup et al., 2001) is identical to the value we report here for NBD-lysoMPE.
The rate constants for spontaneous translocation (flip-flop) of the amphiphiles are very similar for NBD-lysoMPE and NBD-DMPE (Abreu et al., 2004). This fact, taken together with the observed energetics of translocation, implies that the translocation kinetics and thermodynamics are dominated by the same event for both the amphiphiles; this is discussed in greater detail below. It is of interest to note that the translocation rate constant for outer-to-inner monolayer translocation of lyso-1-palmitoylphosphatidylcholine was reported to be 4.4 × 10−5 s−1 in the erythrocyte membrane (Bergmann et al., 1984), a value almost identical to kf for translocation of NBD-lysoMPE across a SpM/Chol (6:4) lo-phase bilayer (see Table 1). This may have to do with the fact that the outer monolayer of the erythrocyte membrane has a significant amount of SpM and Chol and could be predominantly in the lo-phase.
This work and its earlier companion (Abreu et al., 2004) report measured values of all the kinetic constants and their temperature dependence in the association of amphiphiles with lipid bilayer membranes. This permits arriving at a complete mechanistic and thermodynamic description of the association reaction including all of the transition states involved. Such detail, besides providing important insights into the physical properties of the bilayers being studied, has an important predictive value in eventual studies of biological relevance, for example, in the pharmacokinetics of passive amphiphile transport across biological barriers.
There appear to be no surprises in the thermodynamics of the interaction of NBD-lysoMPE when compared with the interaction of NBD-DMPE with the bilayers examined. ΔGo is uniformly ∼10 kJ mol−1 more negative for the interaction of the two-chain probe when compared to its single-chain homolog. This free energy difference is predominantly of enthalpic origin in the case of bilayers prepared from POPC and POPC-Chol (1:1) and predominantly of entropic origin in the case of bilayers prepared from SpM-Chol (6:4). It may be concluded that both probes are poorly solvated in SpM-Chol bilayers as compared with POPC and POPC-Chol bilayers.
The energetics of the activation processes involved in the association of NBD-lysoMPE with the bilayers examined may be understood on the basis of the model previously discussed for NBD-DMPE (Abreu et al., 2004). The desorption of NBD-lysoMPE from the lipid bilayer to the transition state is characterized by a value of ΔG°‡ that is uniformly ∼⅔ of the value that characterized the association of NBD-DMPE with the same three bilayer systems. The enthalpic contribution in this case is considerably lower, and the entropic contribution (a decrease in entropy in all cases) is proportionately significantly higher. The lower value of ΔH°‡ may be explained by the smaller cavities in the transition state necessary for NBD-lysoMPE as compared to NBD-DMPE. The shape mismatch of the probe in the bilayer results in the entropy of the bilayer being higher in the case of NBD-lysoMPE than in the case of NBD-DMPE. In the transition state there is a decrease in entropy that results from the formation of a cavity in the aqueous phase. This is partly compensated for by an increase in entropy when the probe leaves the bilayer to occupy the aqueous phase cavity, leaving a vacant lipid phase cavity. The compensatory effect is larger for NBD-DMPE than it is for NBD-lysoMPE.
The insertion activation process for the single-chain amphiphile is characterized by values of ΔG°‡ that are considerably lower (between ∼36% (POPC and POPC/Chol) and 51% (SpM-Chol)) than the values previously observed for the two-chain homolog. The enthalpic contribution dominates this process in the case of POPC and POPC-Chol, whereas entropy contributes a significant fraction to the transition in the case of SpM-Chol.
In translocation of the amphiphiles from one monolayer of the bilayers to the other, a transition state may be assumed in which both amphiphiles lie in a position somewhere near or at the bilayer midplane and are oriented parallel to the plane of the lipid bilayer. The work that must be done to place the amphiphile in this location is expected to be the work of placing the polar part of the molecule in the hydrophobic bilayer interior. Since both NBD-lysoMPE and NBD-DMPE have almost identical polar headgroups, it is reasonable to expect very similar energetics for the passage to the transition state. In fact,  is almost identical for both probes, very similar for all three bilayers examined, and heavily dominated by the enthalpic component in both cases, as might be expected.
is almost identical for both probes, very similar for all three bilayers examined, and heavily dominated by the enthalpic component in both cases, as might be expected.
In analogy to our previous related work with NBD-DMPE (Abreu et al., 2004), we examine a hypothetical partitioning of NBD-lysoMPE between ld and lo phases or between two lo phases that do not necessarily coexist in the same membrane. The chemical compositions of the membranes used in this work are the three limiting compositions of liquid phases at temperatures below 37°C in the phase diagram of a ternary mixture of SpM, POPC, and Chol. These particular liquids would not coexist in the same membrane if it were constituted from these three chemical constituents. Any determination of KP for membranes with phase coexistence prepared from a given ternary mixture of SpM, POPC, and Chol would have to examine membranes with the corresponding ld and lo phase compositions (read off from the phase diagram) independently and then confirm that the membranes with phase coexistence showed association kinetics that were a simple weighted average of the kinetics for the two coexisting phases. The physical nature and chemical composition of the phase boundaries relative to those of the coexisting phases could, however, result in significant deviations from a simple weighted average (see Pokorny et al., 2000).
In Table 2 we have listed KP and ΔGo for a hypothetical partitioning of NBD-lysoMPE between the membrane phases studied in this work. In comparison with the results previously reported for NBD-DMPE, we note that the difference previously noted between POPC/Chol (1:1) bilayers and SpM/Chol (6:4) bilayers in what concerns partitioning of the probe are significantly less accentuated in the case of association of the present amphiphile with these membranes.
TABLE 2.
Hypothetical equilibrium partition coefficients and thermodynamic parameters for partitioning of NBD-lysoMPE between coexisting lipid phases at 308 K
| Hypothetical system | Type of phase coexistence | KP(L1/L2) |
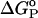 (kJ mol−1) (kJ mol−1) |
|---|---|---|---|
| POPC/POPC-Chol (1:1) | ld/lo | 2.1 | −2 |
| POPC/SpM-Chol (6:4) | ld/lo | 4.7 | −4 |
| POPC-Chol (1:1)/ SpM-Chol (6:4) | lo/lo | 2.3 | −2 |
Acknowledgments
This work was funded in part by research grants from the Portuguese Ministry for Science and Higher Education through the Fundação para a Ciência e a Tecnologia (FCT) via the POCTI program. J.L.S. was supported by a stipend for initiation into scientific research (BIC) through project No. POCTI/38861/BCI/2001 from the FCT.
Abbreviations used: LUV, large unilamellar vesicles with an average diameter of 0.1 μm prepared by an extrusion process; CAC, critical aggregation concentration; Chol, cholesterol; KL, equilibrium association constant for the association of an amphiphile with large unilamellar lipid vesicles; KP, equilibrium partition coefficient; KP(L/W), equilibrium partition coefficient for partitioning between a lipid membrane phase and the aqueous phase; KP(L1/L2), equilibrium partition coefficient for partitioning between two coexisting lipid membrane phases; ld, liquid-disordered; lo, liquid-ordered; NBD-DMPE, N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino-1,2-dimyristoylphosphatidyl-ethanolamine; NBD-lysoMPE, N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino-1-myristoyl-lysophosphatidylethanolamine; POPC, 1-palmitoyl-2-oleoyl phosphatidylcholine; SpM, egg sphingomyelin.
References
- Abrams, F. S., and E. London. 1993. Extension of the parallax analysis of membrane penetration depth to the polar region of model membranes: use of fluorescence quenching by a spin-label attached to the phospholipids polar headgroup. Biochemistry. 32:10826–10831. [DOI] [PubMed] [Google Scholar]
- Abreu, M. S. C., M. J. Moreno, L. M. B. B. Estronca, and W. L. C. Vaz. 2003. Binding of a fluorescent lipid amphiphile to albumin and its transfer to lipid bilayer membranes. Biophys. J. 84:386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu, M. S. C., M. J. Moreno, and W. L. C. Vaz. 2004. Kinetics and thermodynamics of association of a phospholipid derivative with lipid bilayers in liquid-disordered and liquid-Ordered phases. Biophys. J. 87:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniansson, E. A. G., S. N. Wall, M. Almgren, H. Hoffmann, I. Kielmann, W. Ulbricht, R. Zana, J. Lang, and C. Tondre. 1976. Theory and kinetics of micellar equilibria and quantitative interpretation of chemical relaxation studies of micellar solutions of anionic surfactants. J. Phys. Chem. 80:905–922. [Google Scholar]
- Bartlett, G. R. 1959. Phosphorous assay in column chromatography. J. Biol. Chem. 234:466–468. [PubMed] [Google Scholar]
- Bergmann, W. L., V. Dressler, C. W. M. Haest, and B. Deuticke. 1984. Reorientation rates and asymmetry if distribution of lysophospholipids between the inner and outer leaflet of the erythrocyte membrane. Biochim. Biophys. Acta. 772:328–336. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay, A., and E. London. 1987. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry. 26:39–45. [DOI] [PubMed] [Google Scholar]
- Cohen, M. H., and D. Turnbull. 1959. Molecular transport in liquids and glasses. J. Chem. Phys. 31:1164–1169. [Google Scholar]
- Eibl, H. J., J. O. McIntyre, E. A. M. Fleer, and S. Fleischer. 1983. Synthesis of labeled phospholipids in high yield. Methods Enzymol. 98:623–632. [DOI] [PubMed] [Google Scholar]
- Estronca, L. M. B. B., M. J. Moreno, M. S. C. Abreu, E. Melo, and W. L. C. Vaz. 2002. Solubility of amphiphiles in membranes: influence of phase properties and amphiphile headgroup. Biochem. Biophys. Res. Commun. 296:596–603. [DOI] [PubMed] [Google Scholar]
- Estronca, L. M. B. B., M. J. Moreno, J. A. N. Laranjinha, L. M. Almeida, and W. L. C. Vaz. 2005. Kinetics and thermodynamics of lipid amphiphile exchange between lipoproteins and albumin in serum. Biophys. J. 88:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyring, H., and T. Ree. 1961. Significant liquid structures, VI. The vacancy theory of liquids. Proc. Natl. Acad. Sci. USA. 47:527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla, H. J., W. Hartmann, U. Theilen, and E. Sackmann. 1979. On two-dimensional passive random walks in lipid bilayers and fluid pathways in biomembranes. J. Membr. Biol. 48:215–236. [DOI] [PubMed] [Google Scholar]
- Heerklotz, H., and R. M. Epand. 2001. The enthalpy of acyl chain packing and the apparent water-accessible apolar surface area of phospholipids. Biophys. J. 80:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope, M. J., M. B. Bally, G. Webb, and P. R. Cullis. 1985. Production of large unilamellar vesicles by a rapid extrusion procedure—characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta. 812:55–65. [DOI] [PubMed] [Google Scholar]
- Høyrup, P., J. Davidsen, and K. Jørgensen. 2001. Lipid-membrane partitioning of lysolipids and fatty acids: effect of membrane phase structure and detergent chain length. J. Phys. Chem. B. 105:2649–2657. [Google Scholar]
- Huster, D., P. Müller, K. Arnold, and A. Herrmann. 2001. Dynamics of membrane penetration of the fluorescent 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD) group attached to an acyl chain of phosphatidylcholine. Biophys. J. 80:822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, J. C., and R. G. Sleight. 1991. Fluorescence assay for phospholipid membrane asymmetry. Biochemistry. 30:11819–11827. [DOI] [PubMed] [Google Scholar]
- Mesquita, R. M. R. S., E. Melo, T. E. Thompson, and W. L. C. Vaz. 2000. Partitioning of amphiphiles between coexisting ordered and disordered phases in two-phase lipid bilayer membranes. Biophys. J. 78:3019–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, J. W. 1985. Thermodynamics and kinetics of phospholipids monomer-vesicle interaction. Biochemistry. 24:6390–6398. [DOI] [PubMed] [Google Scholar]
- Pokorny, A., P. F. F. Almeida, E. C. C. Melo, and W. L. C. Vaz. 2000. Kinetics of amphiphile association with two-phase lipid bilayer membranes. Biophys. J. 78:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny, A., P. F. F. Almeida, and W. L. C. Vaz. 2001. Association of a fluorescent amphiphile with lipid bilayer vesicles in regions of solid-liquid-disordered phase coexistence. Biophys. J. 80:1384–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker, D. G., and J. W. Nichols. 1990. Hydrophobic interaction of lysophospholipids and bile salts at submicellar concentrations. Biochemistry. 29:5837–5842. [DOI] [PubMed] [Google Scholar]
- Smaby, J. M., M. Momsen, H. L. Brockman, and R. E. Brown. 1997. Phosphatidylcholine acyl unsaturation modulates the decrease in interfacial elasticity induced by cholesterol. Biophys. J. 73:1492–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, R. P., A. V. Broccoli, and C. M. Grisham. 1978. Enzymatic and colorimetric determination of total serum cholesterol. J. Chem. Educ. 55:63–64. [DOI] [PubMed] [Google Scholar]
- Vaz, W. L. C., R. M. Clegg, and D. Hallmann. 1985. Translational diffusion of lipids in liquid-crystalline phase phosphatidylcholine multibilayers: a comparison of experiment with theory. Biochemistry. 26:781–786. [DOI] [PubMed] [Google Scholar]
- Vaz, W. L. C., and E. Melo. 2001. Fluorescence spectroscopic studies of phase heterogeneity in lipid bilayer membranes. J. Fluorescence. 11:255–279. [Google Scholar]
- Vaz, W. L. C., M. J. Moreno, J. L. Sampaio, M. S. C. Abreu, and C. T. A. Vaz. 2004. Kinetics of the Association of Amphiphiles with Lipid Bilayers as a Probe of Membrane Physical State. Biophysical Discussions 2004 of the Biophysical Society.